Page 141 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 141
P1: GNH/MBS P2: GQT Final Pages
Encyclopedia of Physical Science and Technology En012j-597 July 26, 2001 11:8
648 Polymers, Electronic Properties
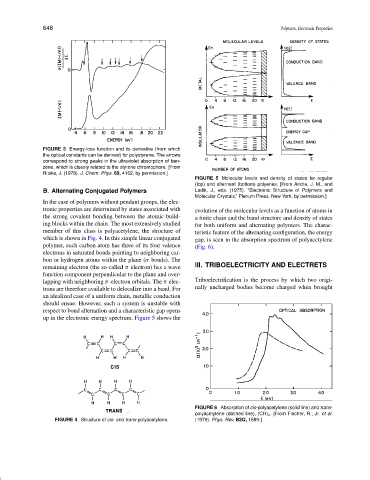
FIGURE 3 Energy-loss function and its derivative (from which
the optical constants can be derived) for polystyrene. The arrows
correspond to strong peaks in the ultraviolet absorption of ben-
zene, which is closely related to the styrene chromophore. [From
Ritsko, J. (1978). J. Chem. Phys. 69, 4162, by permission.]
FIGURE 5 Molecular levels and density of states for regular
(top) and alternant (bottom) polyenes. [From Andre, J. M., and
B. Alternating Conjugated Polymers Ladik, J., eds. (1975). “Electronic Structure of Polymers and
Molecular Crystals,” Plenum Press, New York, by permission.]
In the case of polymers without pendant groups, the elec-
tronic properties are determined by states associated with evolution of the molecular levels as a function of atoms in
the strong covalent bonding between the atomic build- a finite chain and the band structure and density of states
ing blocks within the chain. The most extensively studied for both uniform and alternating polymers. The charac-
member of this class is polyacetylene, the structure of teristic feature of the alternating configuration, the energy
which is shown in Fig. 4. In this simple linear conjugated gap, is seen in the absorption spectrum of polyacetylene
polymer, each carbon atom has three of its four valence (Fig. 6).
electrons in saturated bonds pointing to neighboring car-
bon or hydrogen atoms within the plane (σ bonds). The
remaining electron (the so-called π electron) has a wave III. TRIBOELECTRICITY AND ELECTRETS
function component perpendicular to the plane and over-
lapping with neighboring π-electron orbitals. The π elec- Triboelectrification is the process by which two origi-
trons are therefore available to delocalize into a band. For nally uncharged bodies become charged when brought
an idealized case of a uniform chain, metallic conduction
should ensue. However, such a system is unstable with
respect to bond alternation and a characteristic gap opens
up in the electronic energy spectrum. Figure 5 shows the
FIGURE 6 Absorption of cis-polyacetylene (solid line) and trans-
polyacetylene (dashed line), (CH) x . [From Fincher, R., Jr. et al.
FIGURE 4 Structure of cis- and trans-polyacetylene. (1979). Phys. Rev. B2Q, 1589.]