Page 145 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 145
P1: GNH/MBS P2: GQT Final Pages
Encyclopedia of Physical Science and Technology En012j-597 July 26, 2001 11:8
652 Polymers, Electronic Properties
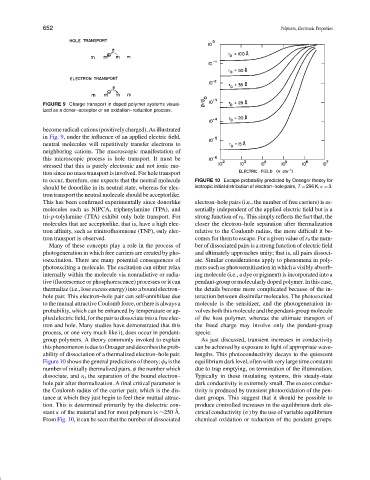
FIGURE 9 Charge transport in doped polymer systems visual-
ized as a donor–acceptor or an oxidation–reduction process.
becomeradical-cations(positivelycharged).Asillustrated
in Fig. 9, under the influence of an applied electric field,
neutral molecules will repetitively transfer electrons to
neighboring cations. The macroscopic manifestation of
this microscopic process is hole transport. It must be
stressed that this is purely electronic and not ionic mo-
tion since no mass transport is involved. For hole transport
to occur, therefore, one expects that the neutral molecule FIGURE 10 Excape probability predicted by Onsager theory for
should be donorlike in its neutral state, whereas for elec- isotropic initial distribution of electron–hole pairs, T = 296 K, κ = 3.
tron transport the neutral molecule should be acceptorlike.
This has been confirmed experimentally since donorlike electron–hole pairs (i.e., the number of free carriers) is es-
molecules such as NIPCA, triphenylamine (TPA), and sentially independent of the applied electric field but is a
tri-p-tolylamine (TTA) exhibit only hole transport. For strong function of r 0 . This simply reflects the fact that, the
molecules that are acceptorlike, that is, have a high elec- closer the electron–hole separation after thermalization
tron affinity, such as trinitrofluorenone (TNF), only elec- relative to the Coulomb radius, the more difficult it be-
tron transport is observed. comes for them to escape. For a given value of r 0 the num-
Many of these concepts play a role in the process of ber of dissociated pairs is a strong function of electric field
photogeneration in which free carriers are created by pho- and ultimately approaches unity; that is, all pairs dissoci-
toexcitation. There are many potential consequences of ate. Similar considerations apply to phenomena in poly-
photoexciting a molecule. The excitation can either relax mers such as photosensitization in which a visibly absorb-
internally within the molecule via nonradiative or radia- ing molecule (i.e., a dye or pigment) is incorporated into a
tive (fluorescence or phosphorescence) processes or it can pendant-group or molecularly doped polymer. In this case,
thermalize (i.e., lose excess energy) into a bound electron– the details become more complicated because of the in-
hole pair. This electron–hole pair can self-annihilate due teraction between dissimilar molecules. The photoexcited
to the mutual attractive Coulomb force, or there is always a molecule is the sensitizer, and the photogeneration in-
probability, which can be enhanced by temperature or ap- volves both this molecule and the pendant-group molecule
plied electric field, for the pair to dissociate into a free elec- of the host polymer, whereas the ultimate transport of
tron and hole. Many studies have demonstrated that this the freed charge may involve only the pendant-group
process, or one very much like it, does occur in pendant- specie.
group polymers. A theory commonly invoked to explain As just discussed, transient increases in conductivity
this phenomenon is due to Onsager and describes the prob- can be achieved by exposure to light of appropriate wave-
ability of dissociation of a thermalized electron–hole pair. lengths. This photoconductivity decays to the quiescent
Figure 10 shows the general predictions of theory; φ 0 is the equilibriumdarklevel,oftenwithverylargetimeconstants
number of initially thermalized pairs, φ the number which due to trap emptying, on termination of the illumination.
dissociate, and r 0 the separation of the bound electron– Typically in these insulating systems, this steady-state
hole pair after thermalization. A final critical parameter is dark conductivity is extremely small. The excess conduc-
the Coulomb radius of the carrier pair, which is the dis- tivity is produced by transient photooxidation of the pen-
tance at which they just begin to feel their mutual attrac- dant groups. This suggest that it should be possible to
tion. This is determined primarily by the dielectric con- produce controlled increases in the equilibrium dark ele-
˚
stant κ of the material and for most polymers is ∼250 A. ctrical conductivity (σ) by the use of variable equilibrium
From Fig. 10, it can be seen that the number of dissociated chemical oxidation or reduction of the pendant groups.