Page 147 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 147
P1: GNH/MBS P2: GQT Final Pages
Encyclopedia of Physical Science and Technology En012j-597 July 26, 2001 11:8
654 Polymers, Electronic Properties
of AsF 5 the band gap is no longer detectable and the
optical properties are those normally associated with a
metal.
The property of polyacetylene that has produced the
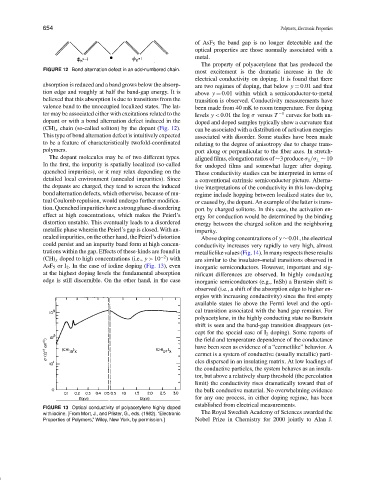
FIGURE 12 Bond alternation defect in an odd-numbered chain.
most excitement is the dramatic increase in the dc
electrical conductivity on doping. It is found that there
absorption is reduced and a band grows below the absorp- are two regimes of doping, that below y = 0.01 and that
tion edge and roughly at half the band-gap energy. It is above y = 0.01 within which a semiconductor-to-metal
believed that this absorption is due to transitions from the transition is observed. Conductivity measurements have
valence band to the unoccupied localized states. The lat- been made from 40 mK to room temperature. For doping
ter may be associated either with excitations related to the levels y < 0.01 the log σ versus T −1 curves for both un-
dopant or with a bond alternation defect induced in the doped and doped samples typically show a curvature that
(CH) x chain (so-called soliton) by the dopant (Fig. 12). can be associated with a distribution of activation energies
This type of bond alternation defect is intuitively expected associated with disorder. Some studies have been made
to be a feature of characteristically twofold-coordinated relating to the degree of anisotropy due to charge trans-
polymers. port along or perpendicular to the fiber axes. In stretch-
The dopant molecules may be of two different types. aligned films, elongation ratios of ∼3 produce σ
/σ ⊥ ∼ 10
In the first, the impurity is spatially localized (so-called for undoped films and somewhat larger after doping.
quenched impurities), or it may relax depending on the These conductivity studies can be interpreted in terms of
detailed local environment (annealed impurities). Since a conventional extrinsic semiconductor picture. Alterna-
the dopants are charged, they tend to screen the induced tive interpretations of the conductivity in this low-doping
bond alternation defects, which otherwise, because of mu- regime include hopping between localized states due to,
tual Coulomb repulsion, would undergo further modifica- or caused by, the dopant. An example of the latter is trans-
tion. Quenched impurities have a strong phase-disordering port by charged solitons. In this case, the activation en-
effect at high concentrations, which makes the Peierl’s ergy for conduction would be determined by the binding
distortion unstable. This eventually leads to a disordered energy between the charged soliton and the neighboring
metallic phase wherein the Peierl’s gap is closed. With an- impurity.
nealed impurities, on the other hand, the Peierl’s distortion Above doping concentrations of y ∼ 0.01, the electrical
could persist and an impurity band form at high concen- conductivity increases very rapidly to very high, almost
trations within the gap. Effects of these-kinds are found in metalliclikevalues(Fig.14).Inmanyrespectstheseresults
−2
(CH) x doped to high concentrations (i.e., y > 10 ) with are similar to the insulator–metal transitions observed in
AsF 5 or I 2 . In the case of iodine doping (Fig. 13), even inorganic semiconductors. However, important and sig-
at the highest doping levels the fundamental absorption nificant differences are observed. In highly conducting
edge is still discernible. On the other hand, in the case inorganic semiconductors (e.g., InSb) a Burstein shift is
observed (i.e., a shift of the absorption edge to higher en-
ergies with increasing conductivity) since the first empty
available states lie above the Fermi level and the opti-
cal transition associated with the band gap remains. For
polyacetylene, in the highly conducting state no Burstein
shift is seen and the band-gap transition disappears (ex-
cept for the special case of I 2 doping). Some reports of
the field and temperature dependence of the conductance
have been seen as evidence of a “cermetlike” behavior. A
cermet is a system of conductive (usually metallic) parti-
cles dispersed in an insulating matrix. At low loadings of
the conductive particles, the system behaves as an insula-
tor, but above a relatively sharp threshold (the percolation
limit) the conductivity rises dramatically toward that of
the bulk conductive material. No overwhelming evidence
for any one process, in either doping regime, has been
established from electrical measurements.
FIGURE 13 Optical conductivity of polyacetylene highly doped
with iodine. [From Mort, J., and Pfister, G., eds. (1982). “Electronic The Royal Swedish Academy of Sciences awarded the
Properties of Polymers,” Wiley, New York, by permission.] Nobel Prize in Chemistry for 2000 jointly to Alan J.