Page 146 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 146
P1: GNH/MBS P2: GQT Final Pages
Encyclopedia of Physical Science and Technology En012j-597 July 26, 2001 11:8
Polymers, Electronic Properties 653
the intrinsically low mobilities associated with the hop-
ping transport mechanism through the localized molecular
states.
VI. CONDUCTING POLYMERS:
POLYACETYLENE
In order for an organic polymer to exhibit high electrical
conductivity in the dark, it must have a high density of free
dark carriers that possess relatively high mobilities. This
requires the polymer to have extended π electrons along
the chain. This can occur either by having aromatic rings
within the chain with large overlap across any atomic link-
ages between the rings or by having unsaturated bonding
within the chain, which also leads to delocalization of π
electrons. The canonical example of the latter type, and
the most extensively studied, is polyacetylene. To put the
previous discussion in perspective, fully saturated poly-
mers such as polyethylene have no π-electron system to
provide the necessary free carriers, although the mobili-
ties in the extended state bands would have the necessary
values. On the other hand, the pendant aromatic groups
in pendant-group polymers have the necessary π-electron
system but an exceeding low intermolecular overlap of
the π orbitals. This is exacerbated by the effect of disor-
der on the orientation of the planar groups and results in
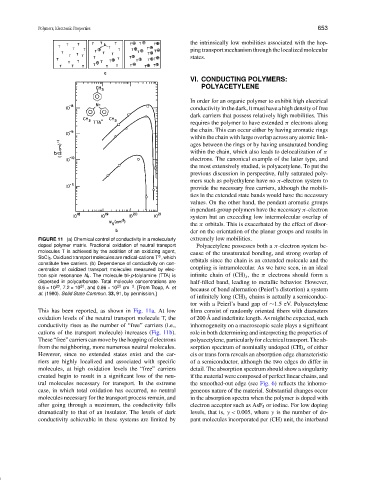
FIGURE 11 (a) Chemical control of conductivity in a molecularly extremely low mobilities.
doped polymer matrix. Fractional oxidation of neutral transport Polyacetylene possesses both a π-electron system be-
molecules T is achieved by the addition of an oxidizing agent,
cause of the unsaturated bonding, and strong overlap of
SbCl 5 . Oxidized transport molecules are radical-cations T , which
⊕
orbitals since the chain is an extended molecule and the
constitute free carriers. (b) Dependence of conductivity on con-
centration of oxidized transport molecules measured by elec- coupling is intramolecular. As we have seen, in an ideal
tron spin resonance N s . The molecule tri-p-tolylamine (TTA) is infinite chain of (CH) x , the π electrons should form a
dispersed in polycarbonate. Total molecule concentrations are half-filled band, leading to metallic behavior. However,
20
20
8.6 × 10 ,7.2 × 10 , and 0.86 × 10 20 cm −3 . [From Troup, A. et because of bond alternation (Peierl’s distortion) a system
al. (1980). Solid State Commun. 33, 91, by permission.]
of infinitely long (CH) x chains is actually a semiconduc-
tor with a Peierl’s band gap of ∼1.5 eV. Polyacetylene
This has been reported, as shown in Fig. 11a. At low films consist of randomly oriented fibers with diameters
˚
oxidation levels of the neutral transport molecule T, the of 200 A and indefinite length. As might be expected, such
conductivity rises as the number of “free” carriers (i.e., inhomogeneity on a macroscopic scale plays a significant
cations of the transport molecule) increases (Fig. 11b). role in both determining and interpreting the properties of
These “free” carriers can move by the hopping of electrons polyacetylene, particularly for electrical transport. The ab-
from the neighboring, more numerous neutral molecules. sorption spectrum of nominally undoped (CH) x of either
However, since no extended states exist and the car- cis or trans form reveals an absorption edge characteristic
riers are highly localized and associated with specific of a semiconductor, although the two edges do differ in
molecules, at high oxidation levels the “free” carriers detail. The absorption spectrum should show a singularity
created begin to result in a significant loss of the neu- if the material were composed of perfect linear chains, and
tral molecules necessary for transport. In the extreme the smoothed-out edge (see Fig. 6) reflects the inhomo-
case, in which total oxidation has occurred, no neutral geneous nature of the material. Substantial changes occur
molecules necessary for the transport process remain, and in the absorption spectra when the polymer is doped with
after going through a maximum, the conductivity falls electron acceptor such as AsF 5 or iodine. For low doping
dramatically to that of an insulator. The levels of dark levels, that is, y < 0.005, where y is the number of do-
conductivity achievable in these systems are limited by pant molecules incorporated per (CH) unit, the interband