Page 148 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 148
P1: GNH/MBS P2: GQT Final Pages
Encyclopedia of Physical Science and Technology En012j-597 July 26, 2001 11:8
Polymers, Electronic Properties 655
maintenance of an absorption edge in the highly doped,
conducting material.
VII. σ-BONDED POLYMERS
Recently, it has been shown that σ-bonded polymers,
which have no conjugated π bonds in their backbone or
side groups, exhibit photoconductive properties and ob-
servable charge transport. Certain polysilane polymers
are known to be photosensitive in the ultraviolet and
indeed are useful as self-developing photoresists. The
σ-electron delocalization plays an important role in the
electronic states of low-molecular-weight polysilanes be-
cause the energy of the σ-σ transition is highly depen-
∗
dent on the polymer conformation. Photogeneration, with
an efficiency of about 1% at high fields, has been shown
to be associated with excition diffusion to the surface.
Dissociation at the surface only leads to the creation of
free holes, since only hole motion is detectable using the
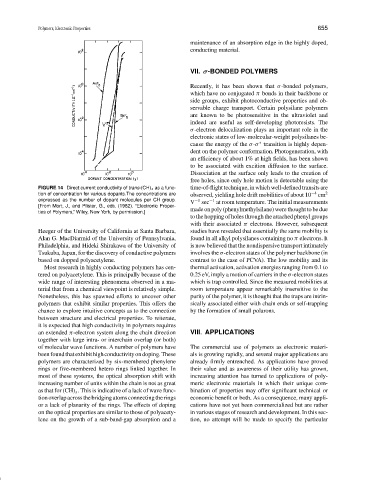
FIGURE 14 Direct current conductivity of trans-(CH) x as a func- time-of-flight technique, in which well-defined transits are
tion of concentration for various dopants.The concentrations are observed, yielding hole drift mobilities of about 10 −4 cm 2
expressed as the number of dopant molecules per CH group. −1 −1
V sec at room temperature. The initial measurements
[From Mort, J., and Pfister, G., eds. (1982). “Electronic Proper-
made on poly (phenylmethylsilane) were thought to be due
ties of Polymers,” Wiley, New York, by permission.]
to the hopping of holes through the attached phenyl groups
with their associated π electrons. However, subsequent
Heeger of the University of California at Santa Barbara, studies have revealed that essentially the same mobility is
Alan G. MacDiarmid of the University of Pennsylvania, found in all alkyl polysilanes containing no π electrons. It
Philadelphia, and Hideki Shirakawa of the University of is now believed that the nondispersive transport intimately
Tsukuba, Japan, for the discovery of conductive polymers involves the σ-electron states of the polymer backbone (in
based on dopped polyacetylene. contrast to the case of PCVA). The low mobility and its
Most research in highly conducting polymers has cen- thermal activation, activation energies ranging from 0.1 to
tered on polyacetylene. This is principally because of the 0.25 eV, imply a motion of carriers in the σ-electron states
wide range of interesting phenomena observed in a ma- which is trap controlled. Since the measured mobilities at
terial that from a chemical viewpoint is relatively simple. room temperature appear remarkably insensitive to the
Nonetheless, this has spawned efforts to uncover other purity of the polymer, it is thought that the traps are intrin-
polymers that exhibit similar properties. This offers the sically associated either with chain ends or self-trapping
chance to explore intuitive concepts as to the connection by the formation of small polarons.
between structure and electrical properties. To reiterate,
it is expected that high conductivity in polymers requires
an extended π-electron system along the chain direction VIII. APPLICATIONS
together with large intra- or interchain overlap (or both)
of molecular wave functions. A number of polymers have The commercial use of polymers as electronic materi-
beenfoundthatexhibithighconductivityondoping.These als is growing rapidly, and several major applications are
polymers are characterized by six-membered phenylene already firmly entrenched. As applications have proved
rings or five-membered hetero rings linked together. In their value and as awareness of their utility has grown,
most of these systems, the optical absorption shift with increasing attention has turned to applications of poly-
increasing number of units within the chain is not as great meric electronic materials in which their unique com-
as that for (CH) x . This is indicative of a lack of wave func- bination of properties may offer significant technical or
tionoverlapacrossthebridgingatomsconnectingtherings economic benefit or both. As a consequence, many appli-
or a lack of planarity of the rings. The effects of doping cations have not yet been commercialized but are rather
on the optical properties are similar to those of polyacety- in various stages of research and development. In this sec-
lene on the growth of a sub-band-gap absorption and a tion, no attempt will be made to specify the particular