Page 144 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 144
P1: GNH/MBS P2: GQT Final Pages
Encyclopedia of Physical Science and Technology En012j-597 July 26, 2001 11:8
Polymers, Electronic Properties 651
is achieved by dissolving the dopant molecule and poly-
mer matrix of appropriate weight ratios in a common sol-
vent. From this solution, films are cast and the solvent
driven off thermally. In the absence of doping, the poly-
carbonate behaves as essentially an ideal insulator with
immeasurably small currents that are unaffected by light
even of ultraviolet energies. In contrast, with the introduc-
tion of NIPCA in concentrations of ∼10 20 molecules per
cubic centimeter, significant photocurrents are observed.
It should be stressed that this system is a true molecular
solid solution; that is, the NIPCA molecules are dispersed
on a molecular level. The photoconductivity excitation
spectrum confirms that the photocarriers are produced by
optical excitation of the NIPCA molecules. By shining
short light pulses, highly absorbed in the film, it is pos-
sible to create a thin sheet of photogenerated charge and,
by monitoring the time evolution of the photocurrent, to
measure the time the sheet of charge (moving under an
applied electric field) takes to traverse the film thickness.
This allows the measurement of a fundamental parame-
ter of the motion of charge: its mobility, µ. By varying
the weight ratios of the NIPCA and polycarbonate, it is
possible to control the average distance between NIPCA
molecules and measure how the mobility depends on this
separation.
Intuitively, in such a system the charge transport is
expected to occur via the hopping of charge from one
molecule to another. This is a quantum mechanical effect
wherein the probability of such an event is determined
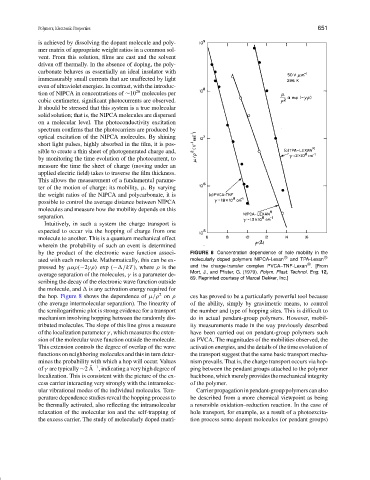
by the product of the electronic wave function associ- FIGURE 8 Concentration dependence of hole mobility in the
ated with each molecule. Mathematically, this can be ex- molecularly doped polymers NIPCA-Lexan and TPA-Lexan
pressed by µαρ(−2γρ) exp (− /kT ), where ρ is the and the charge-transfer complex PVCA–TNF-Lexan . [From
Mort, J., and Pfister, G. (1979). Polym. Plast. Technol. Eng. 12,
average separation of the molecules, γ is a parameter de-
89. Reprinted courtesy of Marcel Dekker, Inc.]
scribing the decay of the electronic wave function outside
the molecule, and is any activation energy required for
2
the hop. Figure 8 shows the dependence of µ/ρ on ρ ces has proved to be a particularly powerful tool because
(the average intermolecular separation). The linearity of of the ability, simply by gravimetric means, to control
the semilogarithmic plot is strong evidence for a transport the number and type of hopping sites. This is difficult to
mechanism involving hopping between the randomly dis- do in actual pendant-group polymers. However, mobil-
tributed molecules. The slope of this line gives a measure ity measurements made in the way previously described
of the localization parameter γ , which measures the exten- have been carried out on pendant-group polymers such
sion of the molecular wave function outside the molecule. as PVCA. The magnitudes of the mobilities observed, the
This extension controls the degree of overlap of the wave activation energies, and the details of the time evolution of
functions on neighboring molecules and this in turn deter- the transport suggest that the same basic transport mecha-
mines the probability with which a hop will occur. Values nism prevails. That is, the charge transport occurs via hop-
˚ −1
of γ are typically ∼2 A , indicating a very high degree of ping between the pendant groups attached to the polymer
localization. This is consistent with the picture of the ex- backbone, which merely provides the mechanical integrity
cess carrier interacting very strongly with the intramolec- of the polymer.
ular vibrational modes of the individual molecules. Tem- Carrierpropagationinpendant-grouppolymerscanalso
perature dependence studies reveal the hopping process to be described from a more chemical viewpoint as being
be thermally activated, also reflecting the intramolecular a reversible oxidation–reduction reaction. In the case of
relaxation of the molecular ion and the self-trapping of hole transport, for example, as a result of a photoexcita-
the excess carrier. The study of molecularly doped matri- tion process some dopant molecules (or pendant groups)