Page 207 - Academic Press Encyclopedia of Physical Science and Technology 3rd Polymer
P. 207
P1: GLM Final Pages
Encyclopedia of Physical Science and Technology EN012c-598 July 26, 2001 15:59
716 Polymers, Mechanical Behavior
a rapid fixed strain is imposed on the material but real- where all chain entanglement effects had been relaxed
ize that this stress would quickly decay due to the fairly out and again the “fish net”or gel structure served as the
rapid flow and sliding of the polymer macromolecules un- resistance for further deformation.
der ambient conditions (it displays a low Deborah num- A final example is that of a cross-linked epoxy ma-
ber). This would lead to a zero stress in a short period terial or glassy network at room temperature. Here one
of time. Similarly, if a fixed load were placed on the would expect relatively little stress relaxation in short
“silly putty” material, we would anticipate that the ma- times, even if un-cross-linked chains existed in the system.
terial would undergo deformation with time (creep) but That is since we are below the glass transition tempera-
not necessarily in a linear fashion. The initial response ture, large-scale cooperative motion cannot occur rapidly
would likely be somewhat elastic, but due to the capac- within the same time frame as in the preceding exper-
ity of these macromolecules to flow at room conditions, iment (a large Deborah number is displayed). With re-
the material dimensions would increase quite rapidly with spect to the creep behavior of this cross-linked glass,
time. one would also expect very little strain to result and
Let us contrast the behavior of the “silly putty” with little change with time for reasons that should be en-
that of a cross-linked rubber band. In stress relaxation one vious. It should then be evident that the tests of stress
would anticipate that some rapid decay in stress would relaxation or creep, coupled with the variables of tem-
occur due to the slipping of chain entanglements as well perature and time, serve as convenient methods with
as any loose chains or loops that make up the network. which to monitor the viscoelastic response of polymeric
However, an equilibrium stress value would be obtained systems.
following the completion of the relaxation of these loose These response times will be influenced accordingly
chain ends, loops, or un-cross-linked chains within the by levels of cross-linking, crystallinity, molecular weight,
system. That is, the final “fish net” or gel macromolecular molecular chain architecture (branched versus linear)
structure would reach an equilibrium stress value at some plasticizer content, and so on, as is clear from the argu-
later time. With respect to the same material response in ments based on wormlike motions. These same variables
creep, one would expect a quick rise in strain due to the should be considered with respect to ultimate properties
imposition of a load, but the strain would then rise much and yield behavior, as well as the low deformation (mod-
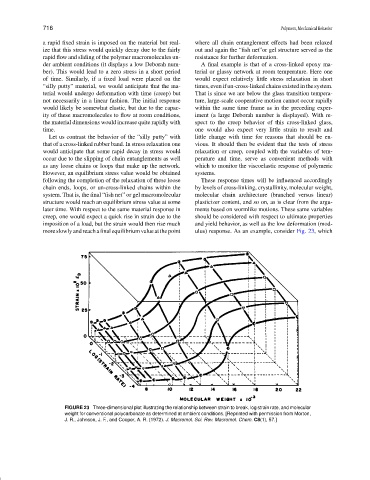
moreslowlyandreachafinalequilibriumvalueatthepoint ulus) response. As an example, consider Fig. 23, which
FIGURE 23 Three-dimensional plot illustrating the relationship between strain to break, log strain rate, and molecular
weight for conventional polycarbonate as determined at ambient conditions. [Reprinted with permission from Morton,
J. R., Johnson, J. F., and Cooper, A. R. (1972). J. Macromol. Sci. Rev. Macromol. Chem. C8(1), 57.]