Page 147 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 147
P1: GLQ/GLT P2: GPJ Final Pages
Encyclopedia of Physical Science and Technology EN007G-334 June 30, 2001 15:23
718 Inclusion (Clathrate) Compounds
cyclodextrin inclusion compounds in 1891, Hofmann’s II. INORGANIC HOSTS
clathrate in 1897, tri-o-thymotide aromatic inclusion
compounds in 1909, Dianin’s compound in 1914, choleic
A. Werner Compounds
acid inclusion compounds in 1916, phenol clathrates in
1935, urea inclusion compounds in 1940, and amylose Werner compounds are named after the coordination
inclusion compounds in 1946. These early studies were chemist who made important contributions to inorganic
hard to understand since the compounds were often non- chemistry at the turn of the century. Specifically, these
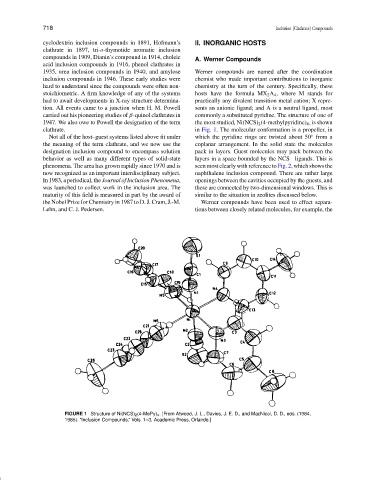
stoichiometric. A firm knowledge of any of the systems hosts have the formula MX 2 A 4 , where M stands for
had to await developments in X-ray structure determina- practically any divalent transition metal cation; X repre-
tion. All events came to a junction when H. M. Powell sents an anionic ligand; and A is a neutral ligand, most
carried out his pioneering studies of β-quinol clathrates in commonly a substituted pyridine. The structure of one of
1947. We also owe to Powell the designation of the term the most studied, Ni(NCS) 2 (4-methylpyridine) 4 , is shown
clathrate. in Fig. 1. The molecular conformation is a propeller, in
◦
Not all of the host–guest systems listed above fit under which the pyridine rings are twisted about 50 from a
the meaning of the term clathrate, and we now use the coplanar arrangement. In the solid state the molecules
designation inclusion compound to encompass solution pack in layers. Guest molecules may pack between the
behavior as well as many different types of solid-state layers in a space bounded by the NCS ligands. This is
−
phenomena. The area has grown rapidly since 1970 and is seen most clearly with reference to Fig. 2, which shows the
now recognized as an important interdisciplinary subject. naphthalene inclusion compound. There are rather large
In 1983, a periodical, the Journal of Inclusion Phenomena, openings between the cavities occupied by the guests, and
was launched to collect work in the inclusion area. The these are connected by two-dimensional windows. This is
maturity of this field is measured in part by the award of similar to the situation in zeolites discussed below.
the Nobel Prize for Chemistry in 1987 to D. J. Cram, J.-M. Werner compounds have been used to effect separa-
Lehn, and C. J. Pedersen. tions between closely related molecules, for example, the
FIGURE 1 Structure of Ni(NCS) 2 (4-MePy) 4 . [From Atwood, J. L., Davies, J. E. D., and MacNicol, D. D., eds. (1984,
1985). “Inclusion Compounds,” Vols. 1–3, Academic Press, Orlando.]