Page 152 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 152
P1: GLQ/GLT P2: GPJ Final Pages
Encyclopedia of Physical Science and Technology EN007G-334 June 30, 2001 15:23
Inclusion (Clathrate) Compounds 723
FIGURE 12 Staging in intercalation reactions of layered host
lattices.
Applications of intercalates have been numerous. Sorp-
tion and ion exchange properties of zeolites and related
substances are regarded under this heading. Catalysis
of the heterogeneous type is emerging on the industrial
level. Hosts with conductivity properties are seeing ac-
tivity in energy storage. On the laboratory scale, new
analytical techniques are being based on this type of
phenomenon.
FIGURE 14 View of the tunnel that results from the packing of
layer type 1 and layer type 2 upon each other.
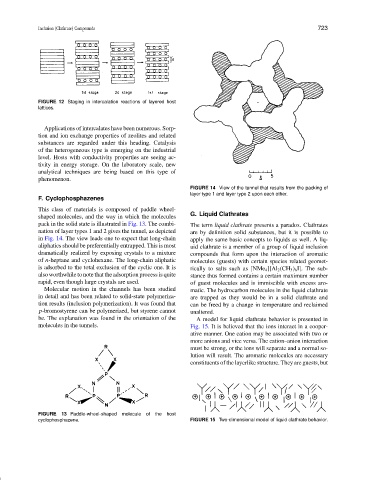
F. Cyclophosphazenes
This class of materials is composed of paddle wheel-
G. Liquid Clathrates
shaped molecules, and the way in which the molecules
pack in the solid state is illustrated in Fig. 13. The combi- The term liquid clathrate presents a paradox. Clathrates
nation of layer types 1 and 2 gives the tunnel, as depicted are by definition solid substances, but it is possible to
in Fig. 14. The view leads one to expect that long-chain apply the same basic concepts to liquids as well. A liq-
aliphatics should be preferentially entrapped. This is most uid clathrate is a member of a group of liquid inclusion
dramatically realized by exposing crystals to a mixture compounds that form upon the interaction of aromatic
of n-heptane and cyclohexane. The long-chain aliphatic molecules (guests) with certain species related geomet-
is adsorbed to the total exclusion of the cyclic one. It is rically to salts such as [NMe 4 ][Al 2 (CH 3 ) 6 I]. The sub-
also worthwhile to note that the adsorption process is quite stance thus formed contains a certain maximum number
rapid, even though large crystals are used. of guest molecules and is immiscible with excess aro-
Molecular motion in the channels has been studied matic. The hydrocarbon molecules in the liquid clathrate
in detail and has been related to solid-state polymeriza- are trapped as they would be in a solid clathrate and
tion results (inclusion polymerization). It was found that can be freed by a change in temperature and reclaimed
p-bromostyrene can be polymerized, but styrene cannot unaltered.
be. The explanation was found in the orientation of the A model for liquid clathrate behavior is presented in
molecules in the tunnels. Fig. 15. It is believed that the ions interact in a cooper-
ative manner. One cation may be associated with two or
more anions and vice versa. The cation–anion interaction
must be strong, or the ions will separate and a normal so-
lution will result. The aromatic molecules are necessary
constituents of the layerlike structure. They are guests, but
FIGURE 13 Paddle-wheel-shaped molecule of the host
cyclophosphazene. FIGURE 15 Two-dimensional model of liquid clathrate behavior.