Page 154 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 154
P1: GLQ/GLT P2: GPJ Final Pages
Encyclopedia of Physical Science and Technology EN007G-334 June 30, 2001 15:23
Inclusion (Clathrate) Compounds 725
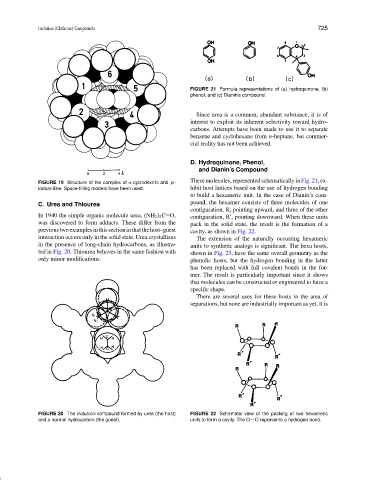
FIGURE 21 Formula representations of (a) hydroquinone, (b)
phenol, and (c) Dianin’s compound.
Since urea is a common, abundant substance, it is of
interest to exploit its inherent selectivity toward hydro-
carbons. Attempts have been made to use it to separate
benzene and cyclohexane from n-heptane, but commer-
cial reality has not been achieved.
D. Hydroquinone, Phenol,
and Dianin’s Compound
These molecules, represented schematically in Fig. 21, ex-
FIGURE 19 Structure of the complex of α-cyclodextrin and p-
iodoaniline. Space-filling models have been used. hibit host lattices based on the use of hydrogen bonding
to build a hexameric unit. In the case of Dianin’s com-
C. Urea and Thiourea pound, the hexamer consists of three molecules of one
configuration, R, pointing upward, and three of the other
In 1940 the simple organic molecule urea, (NH 2 ) 2 C O, configuration, R , pointing downward. When these units
was discovered to form adducts. These differ from the pack in the solid state, the result is the formation of a
previoustwoexamplesinthissectioninthatthehost–guest cavity, as shown in Fig. 22.
interaction occurs only in the solid state. Urea crystallizes The extension of the naturally occurring hexameric
in the presence of long-chain hydrocarbons, as illustra- units to synthetic analogs is significant. The hexa hosts,
ted in Fig. 20. Thiourea behaves in the same fashion with shown in Fig. 23, have the same overall geometry as the
only minor modifications. phenolic hosts, but the hydrogen bonding in the latter
has been replaced with full covalent bonds in the for-
mer. The result is particularly important since it shows
that molecules can be constructed or engineered to have a
specific shape.
There are several uses for these hosts in the area of
separations, but none are industrially important as yet. It is
FIGURE 20 The inclusion compound formed by urea (the host) FIGURE 22 Schematic view of the packing of two hexameric
and a normal hydrocarbon (the guest). units to form a cavity. The O—O represents a hydrogen bond.