Page 155 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 155
P1: GLQ/GLT P2: GPJ Final Pages
Encyclopedia of Physical Science and Technology EN007G-334 June 30, 2001 15:23
726 Inclusion (Clathrate) Compounds
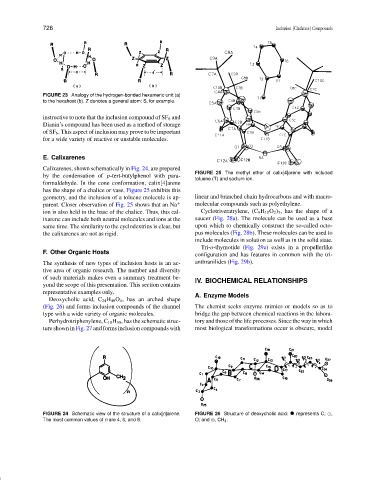
FIGURE 23 Analogy of the hydrogen-bonded hexameric unit (a)
to the hexahost (b). Z denotes a general atom: S, for example.
instructive to note that the inclusion compound of SF 6 and
Dianin’s compound has been used as a method of storage
of SF 6 . This aspect of inclusion may prove to be important
for a wide variety of reactive or unstable molecules.
E. Calixarenes
Calixarenes, shown schematically in Fig. 24, are prepared
FIGURE 25 The methyl ether of calix[4]arene with included
by the condensation of p-tert-butylphenol with para-
toluene (T) and sodium ion.
formaldehyde. In the cone conformation, calix[4]arene
has the shape of a chalice or vase. Figure 25 exhibits this
geometry, and the inclusion of a toluene molecule is ap- linear and branched chain hydrocarbons and with macro-
parent. Closer observation of Fig. 25 shows that an Na + molecular compounds such as polyethylene.
ion is also held in the base of the chalice. Thus, this cal- Cyclotriveratrylene, (C 9 H 10 O 2 ) 3 , has the shape of a
ixarene can include both neutral molecules and ions at the saucer (Fig. 28a). The molecule can be used as a base
same time. The similarity to the cyclodextrins is clear, but upon which to chemically construct the so-called octo-
the calixarenes are not as rigid. pus molecules (Fig. 28b). These molecules can be used to
include molecules in solution as well as in the solid state.
Tri-o-thymotide (Fig. 29a) exists in a propellerlike
F. Other Organic Hosts
configuration and has features in common with the tri-
The synthesis of new types of inclusion hosts is an ac- anthranilides (Fig. 29b).
tive area of organic research. The number and diversity
of such materials makes even a summary treatment be- IV. BIOCHEMICAL RELATIONSHIPS
yond the scope of this presentation. This section contains
representative examples only.
A. Enzyme Models
Deoxycholic acid, C 24 H 40 O 4 , has an arched shape
(Fig. 26) and forms inclusion compounds of the channel The chemist seeks enzyme mimics or models so as to
type with a wide variety of organic molecules. bridge the gap between chemical reactions in the labora-
Perhydrotriphenylene, C 18 H 30 , has the schematic struc- tory and those of the life processes. Since the way in which
ture shown in Fig. 27 and forms inclusion compounds with most biological transformations occur is obscure, model
FIGURE 24 Schematic view of the structure of a calix[n]arene. FIGURE 26 Structure of deoxycholic acid: • represents C;
,
The most common values of n are 4, 6, and 8. O; and ⊗,CH 3 .