Page 153 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 153
P1: GLQ/GLT P2: GPJ Final Pages
Encyclopedia of Physical Science and Technology EN007G-334 June 30, 2001 15:23
724 Inclusion (Clathrate) Compounds
they are also in effect components of the host. The analogy
to certain solid-state inclusion compounds of the Werner
type is to be noted.
Of the several interesting applications of liquid
clathrates is in the area of separations. Since the behav-
ior is found for aromatic molecules but not for aliphatic
ones, a separation is possible. It is possible to envision FIGURE 17 Cryptates. (a) Cryptand [2.1.1], (b) cryptand [2.2.1],
even difficult problems such as the separation of the xy- and (c) cryptand [2.2.2].
lene isomers being attacked by liquid clathrates. Liquid
clathrates have also been reported to be useful as solvents
for the liquefaction of coal.
with specific complexing ability has proved to be an in-
teresting area of synthetic organic chemistry. Since the
III. ORGANIC HOSTS number of naturally occurring organic hosts is limited, im-
portant advances in medicinal chemistry can be expected
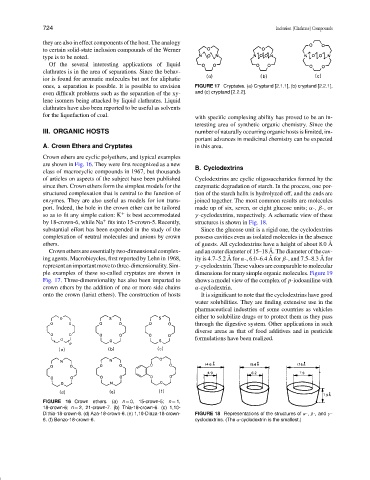
A. Crown Ethers and Cryptates in this area.
Crown ethers are cyclic polyethers, and typical examples
are shown in Fig. 16. They were first recognized as a new
B. Cyclodextrins
class of macrocyclic compounds in 1967, but thousands
of articles on aspects of the subject have been published Cyclodextrins are cyclic oligosaccharides formed by the
since then. Crown ethers form the simplest models for the enzymatic degradation of starch. In the process, one por-
structured complexation that is central to the function of tion of the starch helix is hydrolyzed off, and the ends are
enzymes. They are also useful as models for ion trans- joined together. The most common results are molecules
port. Indeed, the hole in the crown ether can be tailored made up of six, seven, or eight glucose units; α-, β-, or
+
so as to fit any simple cation: K is best accommodated γ -cyclodextrins, respectively. A schematic view of these
+
by 18-crown-6, while Na fits into 15-crown-5. Recently, structures is shown in Fig. 18.
substantial effort has been expended in the study of the Since the glucose unit is a rigid one, the cyclodextrins
complexation of neutral molecules and anions by crown possess cavities even as isolated molecules in the absence
˚
ethers. of guests. All cyclodextrins have a height of about 8.0 A
˚
Crown ethers are essentially two-dimensional complex- and an outer diameter of 15–18 A. The diameter of the cav-
˚
˚
˚
ing agents. Macrobicycles, first reported by Lehn in 1968, ity is 4.7–5.2 A for α-, 6.0–6.4 A for β-, and 7.5–8.3 A for
represent an important move to three-dimensionality. Sim- γ -cyclodextrin. These values are comparable to molecular
ple examples of these so-called cryptates are shown in dimensions for many simple organic molecules. Figure 19
Fig. 17. Three-dimensionality has also been imparted to shows a model view of the complex of p-iodoaniline with
crown ethers by the addition of one or more side chains α-cyclodextrin.
onto the crown (lariat ethers). The construction of hosts It is significant to note that the cyclodextrins have good
water solubilities. They are finding extensive use in the
pharmaceutical industries of some countries as vehicles
either to solubilize drugs or to protect them as they pass
through the digestive system. Other applications in such
diverse areas as that of food additives and in pesticide
formulations have been realized.
FIGURE 16 Crown ethers. (a) n = 0, 15-crown-5; n = 1,
18-crown-6; n = 2, 21-crown-7. (b) Thia-18-crown-6. (c) 1,10-
Dithia-18-crown-6. (d) Aza-18-crown-6. (e) 1,10-Diaza-18-crown- FIGURE 18 Representations of the structures of α-, β-, and γ -
6. (f) Benzo-18-crown-6. cyclodextrins. (The α-cyclodextrin is the smallest.)