Page 148 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 148
P1: GLQ/GLT P2: GPJ Final Pages
Encyclopedia of Physical Science and Technology EN007G-334 June 30, 2001 15:23
Inclusion (Clathrate) Compounds 719
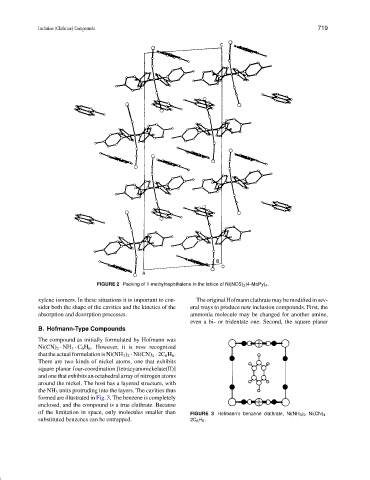
FIGURE 2 Packing of 1-methylnaphthalene in the lattice of Ni(NCS) 2 (4-MePy) 4 .
xylene isomers. In these situations it is important to con- The original Hofmann clathrate may be modified in sev-
sider both the shape of the cavities and the kinetics of the eral ways to produce new inclusion compounds. First, the
absorption and desorption processes. ammonia molecule may be changed for another amine,
even a bi- or tridentate one. Second, the square planar
B. Hofmann-Type Compounds
The compound as initially formulated by Hofmann was
Ni(CN) 2 · NH 3 · C 6 H 6 . However, it is now recognized
that the actual formulation is Ni(NH 3 ) 2 · Ni(CN) 4 · 2C 6 H 6 .
There are two kinds of nickel atoms, one that exhibits
square planar four-coordination [tetracyanonickelate(II)]
and one that exhibits an octahedral array of nitrogen atoms
around the nickel. The host has a layered structure, with
the NH 3 units protruding into the layers. The cavities thus
formed are illustrated in Fig. 3. The benzene is completely
enclosed, and the compound is a true clathrate. Because
of the limitation in space, only molecules smaller than FIGURE 3 Hofmann’s benzene clathrate, Ni(NH 3 ) 2 Ni(CN) 4 ·
substituted benzenes can be entrapped. 2C 6 H 6 .