Page 151 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 151
P1: GLQ/GLT P2: GPJ Final Pages
Encyclopedia of Physical Science and Technology EN007G-334 June 30, 2001 15:23
722 Inclusion (Clathrate) Compounds
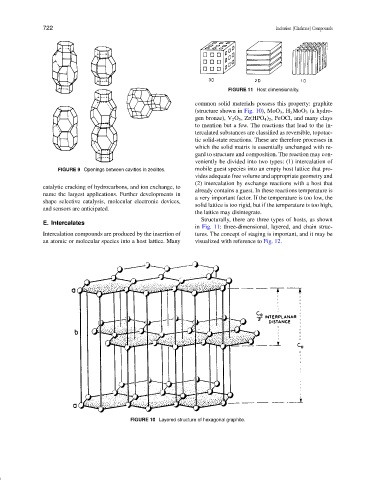
FIGURE 11 Host dimensionality.
common solid materials possess this property: graphite
(structure shown in Fig. 10), MoO 3 , H x MoO 3 (a hydro-
gen bronze), V 2 O 5 , Zr(HPO 4 ) 2 , FeOCl, and many clays
to mention but a few. The reactions that lead to the in-
tercalated substances are classified as reversible, topotac-
tic solid-state reactions. These are therefore processes in
which the solid matrix is essentially unchanged with re-
gard to structure and composition. The reaction may con-
veniently be divided into two types: (1) intercalation of
FIGURE 9 Openings between cavities in zeolites. mobile guest species into an empty host lattice that pro-
vides adequate free volume and appropriate geometry and
(2) intercalation by exchange reactions with a host that
catalytic cracking of hydrocarbons, and ion exchange, to already contains a guest. In these reactions temperature is
name the largest applications. Further developments in
a very important factor. If the temperature is too low, the
shape selective catalysis, molecular electronic devices,
solid lattice is too rigid, but if the temperature is too high,
and sensors are anticipated.
the lattice may disintegrate.
Structurally, there are three types of hosts, as shown
E. Intercalates
in Fig. 11: three-dimensional, layered, and chain struc-
Intercalation compounds are produced by the insertion of tures. The concept of staging is important, and it may be
an atomic or molecular species into a host lattice. Many visualized with reference to Fig. 12.
FIGURE 10 Layered structure of hexagonal graphite.