Page 150 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 150
P1: GLQ/GLT P2: GPJ Final Pages
Encyclopedia of Physical Science and Technology EN007G-334 June 30, 2001 15:23
Inclusion (Clathrate) Compounds 721
at temperatures above the freezing point of water. This
very property has also caused speculation about the use
of the substances as heat-storage media. It is possible to
allow an underground reservoir to freeze in Minnesota and
then use the ice as a source of air conditioning during the
summer. The gas clathrates have nearly the same heat of
fusion as ice, and a reservoir of a high-melting one could
conceivably be used in a like manner in Georgia.
D. Zeolites
Zeolites are porous tectosilicates of typical formulas such
as Li 2 [Al 2 Si 4 O 12 ] · 2H 2 O (bikitaite), Ca 4 [Al 8 Si 28 O 72 ] ·
24H 2 O (heulandite), or (Na 2 ,Ca,Mg) 29 [Al 58 Si 134 O 384 ] ·
240H 2 O. Approximately 60 naturally occurring frame-
work topologies exist, and many new ones have been syn-
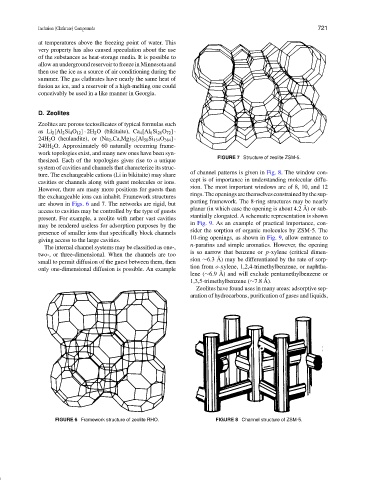
FIGURE 7 Structure of zeolite ZSM-5.
thesized. Each of the topologies gives rise to a unique
system of cavities and channels that characterize its struc-
of channel patterns is given in Fig. 8. The window con-
ture. The exchangeable cations (Li in bikitaite) may share
cept is of importance in understanding molecular diffu-
cavities or channels along with guest molecules or ions.
sion. The most important windows are of 8, 10, and 12
However, there are many more positions for guests than
rings.Theopeningsarethemselvesconstrainedbythesup-
the exchangeable ions can inhabit. Framework structures
porting framework. The 8-ring structures may be nearly
are shown in Figs. 6 and 7. The networks are rigid, but
˚
planar (in which case the opening is about 4.2 A) or sub-
access to cavities may be controlled by the type of guests
stantially elongated. A schematic representation is shown
present. For example, a zeolite with rather vast cavities
in Fig. 9. As an example of practical importance, con-
may be rendered useless for adsorption purposes by the
sider the sorption of organic molecules by ZSM-5. The
presence of smaller ions that specifically block channels
10-ring openings, as shown in Fig. 9, allow entrance to
giving access to the large cavities.
n-parafins and simple aromatics. However, the opening
The internal channel systems may be classified as one-,
is so narrow that benzene or p-xylene (critical dimen-
two-, or three-dimensional. When the channels are too
˚
sion ∼6.3 A) may be differentiated by the rate of sorp-
small to permit diffusion of the guest between them, then
tion from o-xylene, 1,2,4-trimethylbenzene, or naphtha-
only one-dimensional diffusion is possible. An example
˚
lene (∼6.9 A) and will exclude pentamethylbenzene or
˚
1,3,5-trimethylbenzene (∼7.8 A).
Zeolites have found uses in many areas: adsorptive sep-
aration of hydrocarbons, purification of gases and liquids,
FIGURE 6 Framework structure of zeolite RHO. FIGURE 8 Channel structure of ZSM-5.