Page 15 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 15
P1: FYK Revised Pages
Encyclopedia of Physical Science and Technology EN001F-11 May 7, 2001 12:19
Actinide Elements 223
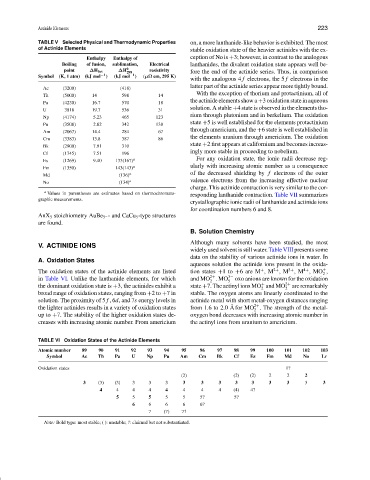
TABLE V Selected Physical and Thermodynamic Properties on, a more lanthanide-like behavior is exhibited. The most
of Actinide Elements stable oxidation state of the heavier actinides with the ex-
Enthalpy Enthalpy of ception of No is +3; however, in contrast to the analogous
Boiling of fusion, sublimation, Electrical lanthanides, the divalent oxidation state appears well be-
point ∆H fus ∆H 0 298 resistivity fore the end of the actinide series. Thus, in comparison
Symbol (K, 1 atm) (kJ mol −1 ) (kJ mol −1 ) (µΩ cm, 295 K)
with the analogous 4 f electrons, the 5 f electrons in the
latter part of the actinide series appear more tightly bound.
Ac (3200) (418)
With the exception of thorium and protactinium, all of
Th (5000) 14 598 14
the actinide elements show a +3 oxidation state in aqueous
Pa (4230) 16.7 570 18
solution. A stable +4 state is observed in the elements tho-
U 3818 19.7 536 31
rium through plutonium and in berkelium. The oxidation
Np (4174) 5.23 465 123
state +5 is well established for the elements protactinium
Pu (3508) 2.82 342 138
through americium, and the +6 state is well established in
Am (2067) 14.4 284 67
the elements uranium through americium. The oxidation
Cm (3383) 13.8 387 86
state +2 first appears at californium and becomes increas-
Bk (2900) 7.91 310
ingly more stable in proceeding to nobelium.
Cf (1745) 7.51 196
Es (1269) 9.40 133(167) a For any oxidation state, the ionic radii decrease reg-
Fm (1350) 143(143) a ularly with increasing atomic number as a consequence
Md (136) a of the decreased shielding by f electrons of the outer
No (134) a valence electrons from the increasing effective nuclear
charge. This actinide contraction is very similar to the cor-
a Values in parentheses are estimates based on thermochromato- responding lanthanide contraction. Table VII summarizes
graphic measurements.
crystallographic ionic radii of lanthanide and actinide ions
for coordination numbers 6 and 8.
AnX 5 stoichiometry AuBe 5 - and CaCu 5 -type structures
−
are found.
B. Solution Chemistry
Although many solvents have been studied, the most
V. ACTINIDE IONS
widely used solvent is still water. Table VIII presents some
data on the stability of various actinide ions in water. In
A. Oxidation States
aqueous solution the actinide ions present in the oxida-
4+
+
The oxidation states of the actinide elements are listed tion states +1to +6 are M ,M ,M ,M ,MO ,
+
2+
3+
2
in Table VI. Unlike the lanthanide elements, for which and MO . MO 3− oxo anions are known for the oxidation
2+
2 5
+
the dominant oxidation state is +3, the actinides exhibit a state +7. The actinyl ions MO and MO 2+ are remarkably
2 2
broad range of oxidation states, ranging from +2to +7in stable. The oxygen atoms are linearly coordinated to the
solution. The proximity of 5 f ,6d, and 7s energy levels in actinide metal with short metal-oxygen distances ranging
˚
2+
the lighter actinides results in a variety of oxidation states from 1.6 to 2.0 A for MO . The strength of the metal-
2
up to +7. The stability of the higher oxidation states de- oxygen bond decreases with increasing atomic number in
creases with increasing atomic number. From americium the actinyl ions from uranium to americium.
TABLE VI Oxidation States of the Actinide Elements
Atomic number 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103
Symbol Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr
Oxidation states 1?
(2) (2) (2) 2 2 2
3 (3) (3) 3 3 3 3 3 3 3 3 3 3 3 3
4 4 4 4 4 4 4 4 (4) 4?
5 5 5 5 5 5? 5?
6 6 6 6 6?
7 (7) 7?
Note: Bold type: most stable; ( ): unstable; ?: claimed but not substantiated.