Page 18 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 18
P1: FYK Revised Pages
Encyclopedia of Physical Science and Technology EN001F-11 May 7, 2001 12:19
226 Actinide Elements
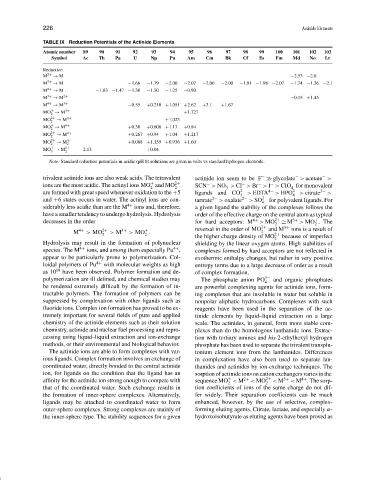
TABLE IX Reduction Potentials of the Actinide Elements
Atomic number 89 90 91 92 93 94 95 96 97 98 99 100 101 102 103
Symbol Ac Th Pa U Np Pu Am Cm Bk Cf Es Fm Md No Lr
Reduction
M 2+ → M −2.53 −2.6
M 3+ → M −1.66 −1.79 −2.00 −2.07 −2.06 −2.00 −1.91 −1.98 −2.07 −1.74 −1.26 −2.1
M 4+ → M −1.83 −1.47 −1.38 −1.30 −1.25 −0.90
M 3+ → M 2+ −0.15 +1.45
M 4+ → M 3+ −0.55 +0.218 +1.051 +2.62 +3.1 +1.67
+
MO → M 3+ +1.727
2
MO 2+ → M 3+ +1.023
2
MO → M 4+ +0.38 +0.606 +1.17 +0.84
+
2
MO 2+ → M 4+ +0.267 +0.94 +1.04 +1.217
2
MO 2+ → M + 2 +0.088 +1.159 +0.936 +1.60
2
+
MO → M 2+ −2.13 +0.04
3 2
Note: Standard reduction potentials in acidic (pH 0) solutions are given in volts vs standard hydrogen electrode.
−
−
−
trivalent actinide ions are also weak acids. The tetravalent actinide ion seem to be F
glycolate > acetate >
+
−
−
−
−
−
−
ions are the most acidic. The actinyl ions MO and MO 2+ SCN > NO > Cl > Br > I > CIO for monovalent
3
2
2
4
are formed with great speed whenever oxidation to the +5 ligands and CO 2− > EDTA 4− > HPO 2− > citrate 3− >
3 4
and +6 states occurs in water. The actinyl ions are con- tartrate 2− > oxalate 2− > SO 2− for polyvalent ligands. For
4
siderably less acidic than are the M 4+ ions and, therefore, a given ligand the stability of the complexes follows the
have a smaller tendency to undergo hydrolysis. Hydrolysis order of the effective charge on the central atom as typical
decreases in the order for hard acceptors: M 4+ > MO 2+ M 3+ > MO . The
+
2 2
reversal in the order of MO 2+ and M 3+ ions is a result of
+
M 4+ > MO 2+ > M 3+ > MO . 2
2 2 2+
the higher charge density of MO because of imperfect
2
Hydrolysis may result in the formation of polynuclear shielding by the linear oxygen atoms. High stabilities of
4+
species. The M 4+ ions, and among them especially Pu , complexes formed by hard acceptors are not reflected in
appear to be particularly prone to polymerization. Col- exothermic enthalpy changes, but rather in very positive
loidal polymers of Pu 4+ with molecular weights as high entropy terms due to a large decrease of order as a result
as 10 10 have been observed. Polymer formation and de- of complex formation.
polymerization are ill defined, and chemical studies may The phosphate anion PO 3− and organic phosphates
4
be rendered extremely difficult by the formation of in- are powerful complexing agents for actinide ions, form-
tractable polymers. The formation of polymers can be ing complexes that are insoluble in water but soluble in
suppressed by complexation with other ligands such as nonpolar aliphatic hydrocarbons. Complexes with such
fluoride ions. Complex ion formation has proved to be ex- reagents have been used in the separation of the ac-
tremely important for several fields of pure and applied tinide elements by liquid–liquid extraction on a large
chemistry of the actinide elements such as their solution scale. The actinides, in general, form more stable com-
chemistry, actinide and nuclear fuel processing and repro- plexes than do the homologous lanthanide ions. Extrac-
cessing using liquid–liquid extraction and ion-exchange tion with tertiary amines and bis-2-ethylhexyl hydrogen
methods, or their environmental and biological behavior. phosphate has been used to separate the trivalent transplu-
The actinide ions are able to form complexes with var- tonium element ions from the lanthanides. Differences
ious ligands. Complex formation involves an exchange of in complexation have also been used to separate lan-
coordinated water, directly bonded to the central actinide thanides and actinides by ion-exchange techniques. The
ion, for ligands on the condition that the ligand has an sorption of actinide ions on cation exchangers varies in the
4+
affinity for the actinide ion strong enough to compete with sequence MO < M 2+ < MO 2+ < M 3+ < M . The sorp-
+
2 2
that of the coordinated water. Such exchange results in tion coefficients of ions of the same charge do not dif-
the formation of inner-sphere complexes. Alternatively, fer widely. Their separation coefficients can be much
ligands may be attached to coordinated water to form enhanced, however, by the use of selective, complex-
outer-sphere complexes. Strong complexes are mainly of forming eluting agents. Citrate, lactate, and especially α-
the inner-sphere type. The stability sequences for a given hydroxoisobutyrate as eluting agents have been proved as