Page 16 - Academic Press Encyclopedia of Physical Science and Technology 3rd InOrganic Chemistry
P. 16
P1: FYK Revised Pages
Encyclopedia of Physical Science and Technology EN001F-11 May 7, 2001 12:19
224 Actinide Elements
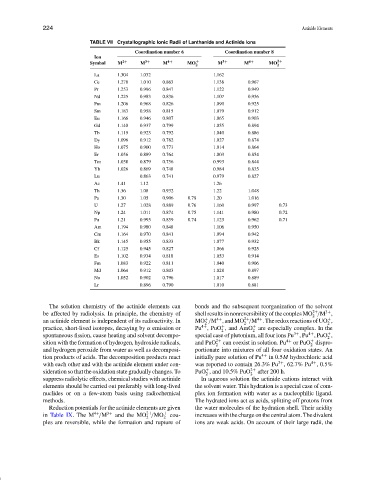
TABLE VII Crystallographic Ionic Radii of Lanthanide and Actinide Ions
Coordination number 6 Coordination number 8
Ion
Symbol M 2+ M 3+ M 4+ MO + M 3+ M 4+ MO 2+
2
2
La 1.304 1.032 1.162
Ce 1.278 1.010 0.863 1.138 0.967
Pr 1.253 0.996 0.847 1.122 0.949
Nd 1.225 0.983 0.836 1.107 0.936
Pm 1.206 0.968 0.826 1.090 0.925
Sm 1.183 0.958 0.815 1.079 0.912
Eu 1.166 0.946 0.807 1.065 0.903
Gd 1.140 0.937 0.799 1.055 0.894
Tb 1.119 0.923 0.792 1.040 0.886
Dy 1.096 0.912 0.782 1.027 0.874
Ho 1.075 0.900 0.773 1.014 0.864
Er 1.056 0.889 0.764 1.003 0.854
Tm 1.038 0.879 0.756 0.993 0.844
Yb 1.026 0.869 0.748 0.984 0.835
Lu 0.863 0.741 0.979 0.827
Ac 1.41 1.12 1.26
Th 1.36 1.08 0.932 1.22 1.048
Pa 1.30 1.05 0.906 0.78 1.20 1.016
U 1.27 1.028 0.889 0.76 1.160 0.997 0.73
Np 1.24 1.011 0.874 0.75 1.141 0.980 0.72
Pu 1.21 0.995 0.859 0.74 1.123 0.962 0.71
Am 1.194 0.980 0.848 1.106 0.950
Cm 1.164 0.970 0.841 1.094 0.942
Bk 1.145 0.955 0.833 1.077 0.932
Cf 1.125 0.945 0.827 1.066 0.925
Es 1.102 0.934 0.818 1.053 0.914
Fm 1.083 0.922 0.811 1.040 0.906
Md 1.064 0.912 0.803 1.028 0.897
No 1.052 0.902 0.796 1.017 0.889
Lr 0.896 0.790 1.010 0.881
The solution chemistry of the actinide elements can bonds and the subsequent reorganization of the solvent
2+
be affected by radiolysis. In principle, the chemistry of shell results in nonreversibility of the couples MO /M ,
3+
2
an actinide element is independent of its radioactivity. In MO /M , and MO /M . The redox reactions of UO ,
+
+
2+
4+
4+
2 2 2
+
practice, short-lived isotopes, decaying by α emission or Pu , PuO , and AmO are especially complex. In the
+
4+
2 2
spontaneous fission, cause heating and solvent decompo- special case of plutonium, all four ions Pu ,Pu , PuO ,
+
3+
4+
2
+
sition with the formation of hydrogen, hydroxide radicals, and PuO 2+ can coexist in solution. Pu 4+ or PuO dispro-
2 2
and hydrogen peroxide from water as well as decomposi- portionate into mixtures of all four oxidation states. An
tion products of acids. The decomposition products react initially pure solution of Pu 4+ in 0.5M hydrochloric acid
with each other and with the actinide element under con- was reported to contain 26.3% Pu , 62.7% Pu , 0.5%
3+
4+
+
sideration so that the oxidation state gradually changes. To PuO , and 10.5% PuO 2+ after 200 h.
2 2
suppress radiolytic effects, chemical studies with actinide In aqueous solution the actinide cations interact with
elements should be carried out preferably with long-lived the solvent water. This hydration is a special case of com-
nuclides or on a few-atom basis using radiochemical plex ion formation with water as a nucleophilic ligand.
methods. The hydrated ions act as acids, splitting off protons from
Reduction potentials for the actinide elements are given the water molecules of the hydration shell. Their acidity
3+ 2+ +
in Table IX. The M /M and the MO /MO cou- increases with the charge on the central atom. The divalent
4+
2 2
ples are reversible, while the formation and rupture of ions are weak acids. On account of their large radii, the