Page 281 - Engineering Plastics Handbook
P. 281
Liquid Crystal Polymer (LCP) 243
aromatic dicarboxylic acids [such as TPA, isophthalic acid (IPA), and
2,6-dicarboxynaphthalene (NDA)].
2. Acidolysis-type polycondensation reaction using aromatic hydroxyl
compounds (such as HBA, HNA, and DHB), aromatic dicarboxylic
acids (TPA and/or IPA), and acetic anhydride.
3. Acidolysis-type polycondensation reaction using acetyl derivative of
HBA and PET or PET oligomers.
® ®
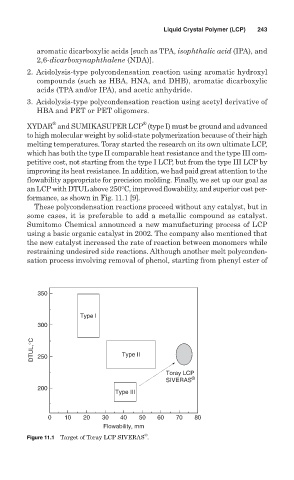
XYDAR and SUMIKASUPER LCP (type I) must be ground and advanced
to high molecular weight by solid-state polymerization because of their high
melting temperatures. Toray started the research on its own ultimate LCP,
which has both the type II comparable heat resistance and the type III com-
petitive cost, not starting from the type I LCP, but from the type III LCP by
improving its heat resistance. In addition, we had paid great attention to the
flowability appropriate for precision molding. Finally, we set up our goal as
an LCPwith DTULabove 250°C, improved flowability, and superior cost per-
formance, as shown in Fig. 11.1 [9].
These polycondensation reactions proceed without any catalyst, but in
some cases, it is preferable to add a metallic compound as catalyst.
Sumitomo Chemical announced a new manufacturing process of LCP
using a basic organic catalyst in 2002. The company also mentioned that
the new catalyst increased the rate of reaction between monomers while
restraining undesired side reactions. Although another melt polyconden-
sation process involving removal of phenol, starting from phenyl ester of
350
Type I
300
DTUL,°C 250 Type II
Toray LCP
SIVERAS ®
200
Type III
0 10 20 30 40 50 60 70 80
Flowability, mm
®
Figure 11.1 Target of Toray LCP SIVERAS .