Page 385 - Enhanced Oil Recovery in Shale and Tight Reservoirs
P. 385
Fracturing fluid flow back 357
when p g is reduced, y w must be increased. So water must be vaporized as the
gas phase pressure is decreased.
However, when more water is vaporized, the salt concentration in the
aqueous phase will be increased. As a result, x w will be decreased. Then
the water vaporization will be reduced (Morin and Montel, 1995). Whether
more water is vaporized depends on the competitive effect of pressure
reduction and the effect of salinity increase. In real gas flow or petroleum
problems, the effect of pressure reduction should be more important.
When salt precipitation starts to occur, the salinity becomes a constant. Wa-
ter is vaporized until no water exists, as the pressure declines.
In fracturing shale reservoirs, it has been observed that less water is pro-
duced than the pumped fracturing fluid in some cases. Water vaporization is
partly attributed to the phenomenon. However, Fig. 12.13 shows that the
mole fractions of water vapor in the gas phase changes near 0.01 with a large
pressure interval. In other words, even though the pressure changes signifi-
cantly, the mole fraction in the gas phase remains very small. Then the water
vaporization due to pressure drawdown should not be significant, if the gas is
saturated with water initially.
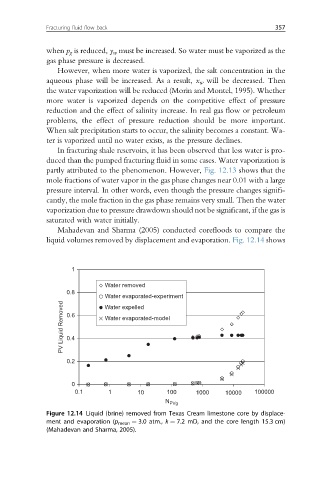
Mahadevan and Sharma (2005) conducted corefloods to compare the
liquid volumes removed by displacement and evaporation. Fig. 12.14 shows
1
Water removed
0.8
Water evaporated-experiment
PV Liquid Removed 0.6 Water evaporated-model
Water expelled
0.4
0.2
0
0.1 1 10 100 1000 10000 100000
N PVg
Figure 12.14 Liquid (brine) removed from Texas Cream limestone core by displace-
ment and evaporation (p mean ¼ 3.0 atm., k ¼ 7.2 mD, and the core length 15.3 cm)
(Mahadevan and Sharma, 2005).