Page 384 - Enhanced Oil Recovery in Shale and Tight Reservoirs
P. 384
356 Enhanced Oil Recovery in Shale and Tight Reservoirs
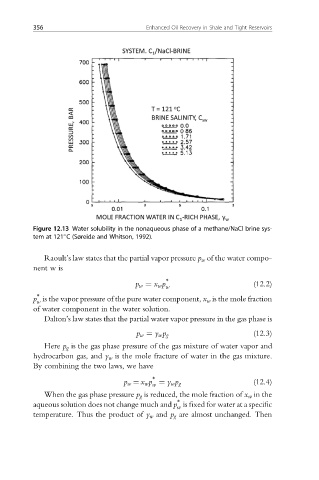
Figure 12.13 Water solubility in the nonaqueous phase of a methane/NaCl brine sys-
tem at 121 C (Søreide and Whitson, 1992).
Raoult’s law states that the partial vapor pressure p w of the water compo-
nent w is
*
p w ¼ x w p w (12.2)
*
p is the vapor pressure of the pure water component, x w is the mole fraction
w
of water component in the water solution.
Dalton’s law states that the partial water vapor pressure in the gas phase is
p w ¼ y w p g (12.3)
Here p g is the gas phase pressure of the gas mixture of water vapor and
hydrocarbon gas, and y w is the mole fracture of water in the gas mixture.
By combining the two laws, we have
*
p w ¼ x w p ¼ y w p g (12.4)
w
When the gas phase pressure p g is reduced, the mole fraction of x w in the
*
aqueous solution does not change much and p is fixed for water at a specific
w
temperature. Thus the product of y w and p g are almost unchanged. Then