Page 115 - Entrophy Analysis in Thermal Engineering Systems
P. 115
108 Entropy Analysis in Thermal Engineering Systems
2 3
Λ
3:76Λ
a a
1 6 y O 2 y N 2 7
p f
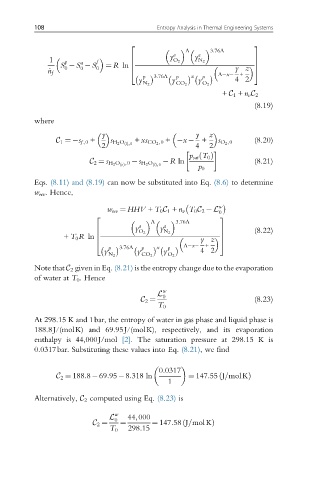
a ¼ R ln
0
7
0
S S S 0 6 y z 5
4
3:76Λ Λ x +
_ n f
4 2
p p x p
N 2 CO 2 O 2
y y y
+ C 1 + n v C 2
(8.19)
where
C 1 ¼ s f ,0 + y s H 2 O lðÞ,0 + xs CO 2 ,0 + x + z s O 2 ,0 (8.20)
y
2 4 2
ð
p sat T 0 Þ
R ln (8.21)
C 2 ¼ s H 2 O vðÞ ,0 s H 2 O lðÞ,0
p 0
Eqs. (8.11) and (8.19) can now be substituted into Eq. (8.6) to determine
w rev . Hence,
w rev ¼ HHV + T 0 C 1 + n v T 0 C 2 L w
0
2 3
Λ
3:76Λ
a a
y y (8.22)
O 2 N 2
6 7
+ T 0 R ln 6 7
4 y z 5
3:76Λ Λ x +
4 2
p p x p
y y y
N 2 CO 2 O 2
Note that C 2 given in Eq. (8.21) is the entropy change due to the evaporation
of water at T 0 . Hence
L w
0
C 2 ¼ (8.23)
T 0
At 298.15 K and 1bar, the entropy of water in gas phase and liquid phase is
188.8J/(molK) and 69.95J/(molK), respectively, and its evaporation
enthalpy is 44,000J/mol [2]. The saturation pressure at 298.15 K is
0.0317bar. Substituting these values into Eq. (8.21), we find
0:0317
C 2 ¼ 188:8 69:95 8:318 ln ¼ 147:55 J=molKÞ
ð
1
Alternatively, C 2 computed using Eq. (8.23) is
L w 44;000
0
C 2 ¼ ¼ ¼ 147:58 J=mol KÞ
ð
T 0 298:15