Page 49 - Entrophy Analysis in Thermal Engineering Systems
P. 49
40 Entropy Analysis in Thermal Engineering Systems
p 2
T H,1
3a
I
4a
T H,2
1 3
T L II
4a¢
T L 4
V
Fig. 3.6 Illustration of the cycle of Fig. 3.5 with two Carnot cycles designated as I and II.
Q H,2 Q L,II
+ ¼ 0 (3.24)
T H,2 T L
Adding Eqs. (3.23) and (3.24) leads to Eq. (3.22).
A similar analysis can be made for a Carnot-like cycle, which receives
heat from a single heat source but rejects heat to two low-temperature res-
ervoirs. As depicted in Fig. 3.7, the cycle consists of two adiabatic compres-
sion, two isothermal cooling, one isothermal heating, and one adiabatic
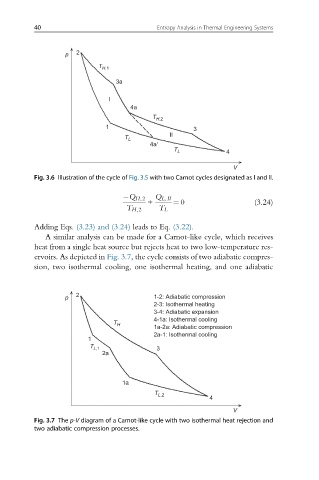
p 2 1-2: Adiabatic compression
2-3: Isothermal heating
3-4: Adiabatic expansion
T H 4-1a: Isothermal cooling
1a-2a: Adiabatic compression
2a-1: Isothermal cooling
1
T L,1 3
2a
1a
T L,2
4
V
Fig. 3.7 The p-V diagram of a Carnot-like cycle with two isothermal heat rejection and
two adiabatic compression processes.