Page 58 - Entrophy Analysis in Thermal Engineering Systems
P. 58
50 Entropy Analysis in Thermal Engineering Systems
The negative sign indicates that the heat is lost from the source. The total
entropy generation associated with isothermal expansion of an ideal gas is
obtained as follows.
ð
Φ ¼ nR ln V f δQ (4.15)
T s
V i
4.5 Mixing
A further widely known process that leads to a production of entropy
is mixing two or more fluids. We will first consider mixing of liquids and
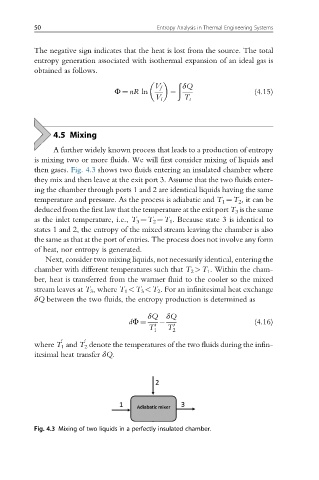
then gases. Fig. 4.3 shows two fluids entering an insulated chamber where
they mix and then leave at the exit port 3. Assume that the two fluids enter-
ing the chamber through ports 1 and 2 are identical liquids having the same
temperature and pressure. As the process is adiabatic and T 1 ¼T 2 , it can be
deduced from the first law that the temperature at the exit port T 3 is the same
as the inlet temperature, i.e., T 3 ¼T 2 ¼T 1 . Because state 3 is identical to
states 1 and 2, the entropy of the mixed stream leaving the chamber is also
the same as that at the port of entries. The process does not involve any form
of heat, nor entropy is generated.
Next, consider two mixing liquids, not necessarily identical, entering the
chamber with different temperatures such that T 2 >T 1 . Within the cham-
ber, heat is transferred from the warmer fluid to the cooler so the mixed
stream leaves at T 3 , where T 1 <T 3 <T 2 . For an infinitesimal heat exchange
δQ between the two fluids, the entropy production is determined as
δQ δQ
dΦ ¼ (4.16)
0 0
T 1 T 2
0 0
where T 1 and T 2 denote the temperatures of the two fluids during the infin-
itesimal heat transfer δQ.
Fig. 4.3 Mixing of two liquids in a perfectly insulated chamber.