Page 59 - Entrophy Analysis in Thermal Engineering Systems
P. 59
The common source of entropy increase 51
0 0
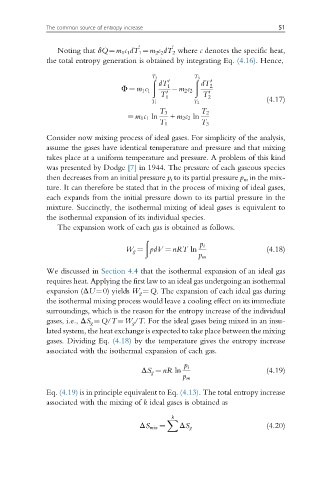
Noting that δQ¼m 1 c 1 dT 1 ¼m 2 c 2 dT 2 where c denotes the specific heat,
the total entropy generation is obtained by integrating Eq. (4.16). Hence,
ð 3 ð 3
T T
0 0
dT 1 dT 2
Φ ¼ m 1 c 1 m 2 c 2
0 0
1 2
T T (4.17)
T 1 T 2
T 3 T 2
¼ m 1 c 1 ln + m 2 c 2 ln
T 1 T 3
Consider now mixing process of ideal gases. For simplicity of the analysis,
assume the gases have identical temperature and pressure and that mixing
takes place at a uniform temperature and pressure. A problem of this kind
was presented by Dodge [7] in 1944. The pressure of each gaseous species
then decreases from an initial pressure p i to its partial pressure p m in the mix-
ture. It can therefore be stated that in the process of mixing of ideal gases,
each expands from the initial pressure down to its partial pressure in the
mixture. Succinctly, the isothermal mixing of ideal gases is equivalent to
the isothermal expansion of its individual species.
The expansion work of each gas is obtained as follows.
ð
W g ¼ pdV ¼ nRT ln p m (4.18)
p i
We discussed in Section 4.4 that the isothermal expansion of an ideal gas
requires heat. Applying the first law to an ideal gas undergoing an isothermal
expansion (ΔU¼0) yields W g ¼Q. The expansion of each ideal gas during
the isothermal mixing process would leave a cooling effect on its immediate
surroundings, which is the reason for the entropy increase of the individual
gases, i.e., ΔS g ¼Q/T¼W g /T. For the ideal gases being mixed in an insu-
lated system, the heat exchange is expected to take place between the mixing
gases. Dividing Eq. (4.18) by the temperature gives the entropy increase
associated with the isothermal expansion of each gas.
ΔS g ¼ nR ln p m (4.19)
p i
Eq. (4.19) is in principle equivalent to Eq. (4.13). The total entropy increase
associated with the mixing of k ideal gases is obtained as
k
X
ΔS mix ¼ ΔS g (4.20)