Page 173 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 173
158 Principles and Methods
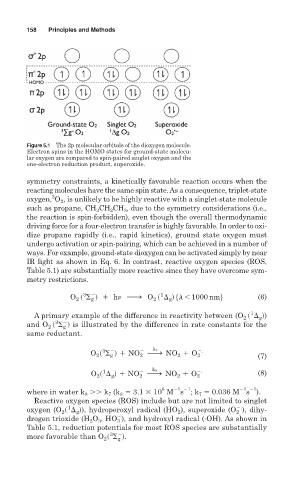
Figure 5.1 The 2p molecular orbitals of the dioxygen molecule.
Electron spins in the HOMO states for ground-state molecu-
lar oxygen are compared to spin-paired singlet oxygen and the
one-electron reduction product, superoxide.
symmetry constraints, a kinetically favorable reaction occurs when the
reacting molecules have the same spin state. As a consequence, triplet-state
3
oxygen, O 2 , is unlikely to be highly reactive with a singlet-state molecule
such as propane, CH 3 CH 2 CH 3 , due to the symmetry considerations (i.e.,
the reaction is spin-forbidden), even though the overall thermodynamic
driving force for a four-electron transfer is highly favorable. In order to oxi-
dize propane rapidly (i.e., rapid kinetics), ground state oxygen must
undergo activation or spin-pairing, which can be achieved in a number of
ways. For example, ground-state dioxygen can be activated simply by near
IR light as shown in Eq. 6. In contrast, reactive oxygen species (ROS,
Table 5.1) are substantially more reactive since they have overcome sym-
metry restrictions.
3 2 1
O s g d 1 hn h O s d 5l ,1000 nm6 (6)
2
2
g
1
A primary example of the difference in reactivity between (O s d )
g
2
3 2
and O s g d is illustrated by the difference in rate constants for the
2
same reductant.
3 2 2 k 7 2.
O s g d 1 NO 2 h NO 1 O 2 (7)
2
2
1 2 k 8 2.
O s g d 1 NO 2 h NO 2 1 O 2 (8)
2
6 1 1 1 1
k (k 3.1
10 M s ; k 0.036 M s ).
where in water k 8 7 8 7
Reactive oxygen species (ROS) include but are not limited to singlet
1
oxygen (O s d ), hydroperoxyl radical (HO 2 . ), superoxide (O 2 2. ), dihy-
2
g
2
2
3
drogen trioxide (H O , HO 3 ), and hydroxyl radical ( OH). As shown in
Table 5.1, reduction potentials for most ROS species are substantially
3 2
more favorable than O 2 s g . d