Page 177 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 177
162 Principles and Methods
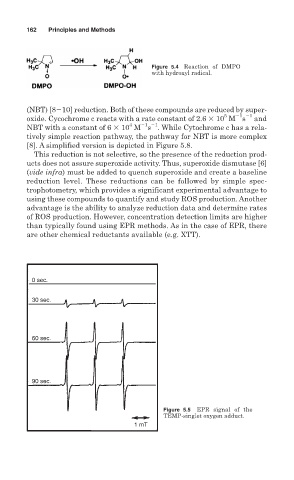
Figure 5.4 Reaction of DMPO
with hydroxyl radical.
(NBT) [8 10] reduction. Both of these compounds are reduced by super-
1 1
5
oxide. Cycochrome c reacts with a rate constant of 2.6
10 M s and
1 1
4
NBT with a constant of 6
10 M s . While Cytochrome c has a rela-
tively simple reaction pathway, the pathway for NBT is more complex
[8]. A simplified version is depicted in Figure 5.8.
This reduction is not selective, so the presence of the reduction prod-
ucts does not assure superoxide activity. Thus, superoxide dismutase [6]
(vide infra) must be added to quench superoxide and create a baseline
reduction level. These reductions can be followed by simple spec-
trophotometry, which provides a significant experimental advantage to
using these compounds to quantify and study ROS production. Another
advantage is the ability to analyze reduction data and determine rates
of ROS production. However, concentration detection limits are higher
than typically found using EPR methods. As in the case of EPR, there
are other chemical reductants available (e.g. XTT).
0 sec.
30 sec.
60 sec.
90 sec.
Figure 5.5 EPR signal of the
TEMP-singlet oxygen adduct.
1 mT