Page 182 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 182
Reactive Oxygen Species Generation on Nanoparticulate Material 167
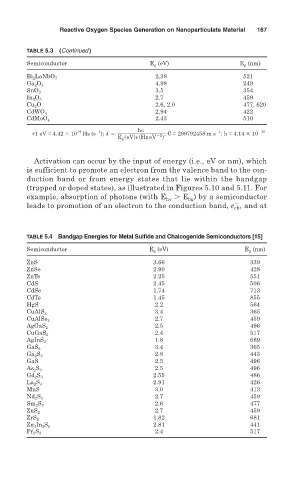
TABLE 5.3 (Continued)
Semiconductor E g (eV) E g (nm)
Bi 2 LaNbO 7 2.38 521
4.98 249
Ga 2 O 3
SnO 2 3.5 354
2.7 459
In 2 O 3
Cu 2 O 2.6, 2.0 477, 620
2.94 422
CdWO 4
2.43 510
CdMoO 4
1
1
14
∗1 eV = 4.42
10 Hz (s ); l 5 hc 21 ; C = 299792458 m s ; h = 4.14
10 15
E g seVdnsHz eV d
Activation can occur by the input of energy (i.e., eV or nm), which
is sufficient to promote an electron from the valence band to the con-
duction band or from energy states that lie within the bandgap
(trapped or doped states), as illustrated in Figures 5.10 and 5.11. For
example, absorption of photons (with E hv E bg ) by a semiconductor
2
leads to promotion of an electron to the conduction band, e cb , and at
TABLE 5.4 Bandgap Energies for Metal Sulfide and Chalcogenide Semiconductors [15]
Semiconductor E g (eV) E g (nm)
ZnS 3.66 339
ZnSe 2.90 428
ZnTe 2.25 551
CdS 2.45 506
CdSe 1.74 713
CdTe 1.45 855
HgS 2.2 564
3.4 365
CuAlS 2
2.7 459
CuAlSe 2
2.5 496
AgGaS 2
2.4 517
CuGaS 2
1.8 689
AgInS 2
3.4 365
GaS 2
2.8 443
Ga 2 S 3
GaS 2.5 496
2.5 496
As 2 S 3
2.55 486
Gd 2 S 3
2.91 426
La 2 S 3
MnS 3.0 413
2.7 459
Nd 2 S 3
2.6 477
Sm 2 S 3
2.7 459
ZnS 2
1.82 681
ZrS 2
2.81 441
Zn 3 In 2 S 6
2.4 517
Pr 2 S 3