Page 184 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 184
Reactive Oxygen Species Generation on Nanoparticulate Material 169
hn
(a)
+ +
∗ +
+
(a) +
(b)
(b)
(c)
+ + (d)
A – + +
D
TiO 2
+
–
A + e → A – D + h → D +
A D
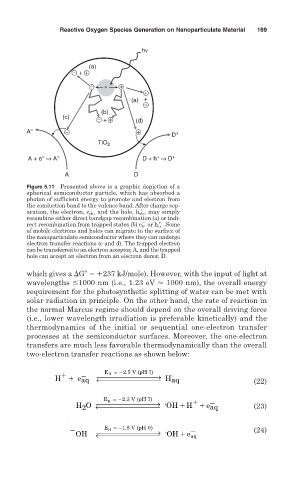
Figure 5.11 Presented above is a graphic depiction of a
spherical semiconductor particle, which has absorbed a
photon of sufficient energy to promote and electron from
the conduction band to the valence band. After charge sep-
2 1
aration, the electron, e cb , and the hole, h vb , may simply
recombine either direct bandgap recombination (a) or indi-
2 1
rect recombination from trapped states (b) e tr or h tr . Some
of mobile electrons and holes can migrate to the surface of
the nanoparticulate semiconductor where they can undergo
electron transfer reactions (c and d). The trapped electron
can be transferred to an electron acceptor, A, and the trapped
hole can accept an electron from an electron donor, D.
o
which gives a G 237 kJ/mole). However, with the input of light at
wavelengths 1000 nm (i.e., 1.23 eV 1000 nm), the overall energy
requirement for the photosynthetic splitting of water can be met with
solar radiation in principle. On the other hand, the rate of reaction in
the normal Marcus regime should depend on the overall driving force
(i.e., lower wavelength irradiation is preferable kinetically) and the
thermodynamics of the initial or sequential one-electron transfer
processes at the semiconductor surfaces. Moreover, the one-electron
transfers are much less favorable thermodynamically than the overall
two-electron transfer reactions as shown below:
2.5 V (pH 7)
E H
⎯⎯⎯⎯⎯⎯⎯⎯ .
→
⎯
H e aq ←⎯⎯⎯⎯⎯⎯⎯ H aq (22)
2.3 V (pH 7)
E
→
⎯⎯⎯⎯⎯⎯⎯⎯
H
⎯
H O ←⎯⎯⎯⎯⎯⎯⎯ . OH H e q (23)
aq
2
1.8 V (pH 0)
⎯⎯⎯⎯⎯⎯⎯⎯ . (24)
→
E H
⎯
OH ←⎯⎯⎯⎯⎯⎯⎯ OH e aq