Page 185 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 185
170 Principles and Methods
In the presence of oxygen, the reductive process in water leading to
2.
superoxide ion, O 2 has the following half-reaction and a reduction
potential of E 0.33 V: at pH 7.
H
E H 520.33V
2.
2
O 1 e m O 2 (25)
pH57
On the oxidative side, the water or hydroxyl radical has corresponding
potential at pH 0:
E H 521.8V
2 OH m . 2 (26)
pH57 OH 1 e aq
However, when working with hydrated metal oxide surfaces one must
take into account the nature of the reactive surface sites that normally
involve metal hydroxyl functionalities. In the specific case of dehydrated
TiO (see Figure 5.13), we must consider that when exposed to water
2
either in a humid atmosphere or in aqueous suspension the surface
titanium-oxygen bonds in the crystallites undergo hydrolysis to pro-
duce surface hydroxyl groups as follows [16 27]:
k h
. Ti-O-Ti ,1 H O m 2 . TiOH (27)
2
k d
The metal hydroxyl surface sites (e.g., TiOH or FeOH) exist under
ambient conditions in an open atmosphere or in an aqueous suspension.
Once hydrolyzed, the surface hydroxyl groups either gain or lose a proton
eV
0 Vacuum level E vs. NHE
–3.0
–1.0
–4.0 3.0
–4.5 1.1 0 H /H +
2
–5.0 2.3 1.7 2.5
Si +1.0
/H O
O 2 2
–6.0 3.2 2.7 2.8 3.2 5.0 3.4 2.8 2.2 3.2 3.8 3.4 3.2 3.0
GaP CdSe +2.0
SiC
–7.0
CdS
Fe O 3 +3.0
2
–8.0
SrTiO MnTiO ZnO TiO
3 3 WO KTaO 2
FeTiO BaTiO Nb O 5 3 3 Rutile
2
3 3
TiO
2
ZrO Anatase
2
SnO 2
pH = 0
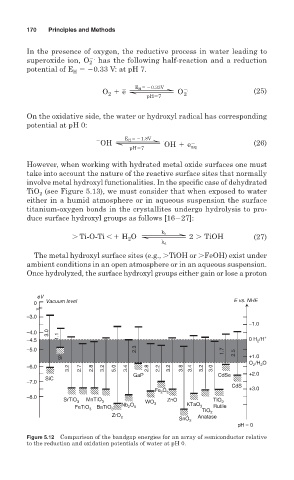
Figure 5.12 Comparison of the bandgap energies for an array of semiconductor relative
to the reduction and oxidation potentials of water at pH 0.