Page 186 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 186
Reactive Oxygen Species Generation on Nanoparticulate Material 171
depending on the intrinsic acidity of the metal oxide [24, 28]. A simple
formalism for characterizing the surface acidity versus pH, for quanti-
fying the surface buffering capacity, the surface ion-exchange properties,
and surface complexation capacity for cations, anions, and ligands is
presented in Eqs. 28 and 29. The pH dependent changes in terms of the
acid-base chemistry of surface hydroxyl functionalities (e.g., MOH,
TiOH, FeOH) can be treated as a conventional diprotic acid, although
there may be more than one type of surface site undergoing protonation
s
and deprotonation (i.e., a distribution of surface acidity constants, K a .
, the titration of a colloidal sus-
In the case of nanoparticulate TiO 2
pension with NaOH gives a classical titration curve for a diprotic acid
as shown in Figure 5.14. Using the titration data of Figure 5.14, the sur-
face acidity of TiO can be characterized is terms of two surface acidity
2
constants as follows:
1
k 1
, TiOH m , TiOH 1 H 1
2
k 21
(28)
k 1
s
s
K al 5 pK al 5 2.4
k
21
k 2 2 1
m , TiO 1 H
, TiOH
k 22
(29)
s
s
K a2 5 k 2 pK a2 5 8.0
k
22
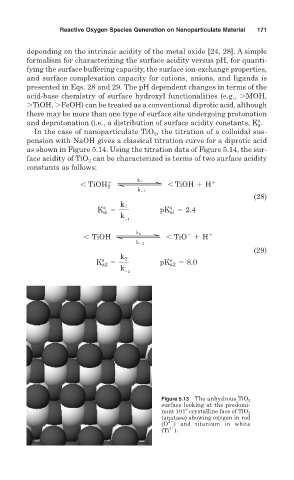
Figure 5.13 The anhydrous TiO 2
surface looking at the predomi-
nant 101” crystalline face of TiO 2
(anatase) showing oxygen in red
2
(O ) and titanium in white
4
(Ti ).