Page 183 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 183
168 Principles and Methods
Energy
hn
Conduction band
∆E g
+
Valence band
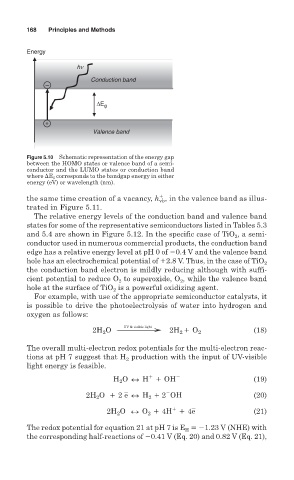
Figure 5.10 Schematic representation of the energy gap
between the HOMO states or valence band of a semi-
conductor and the LUMO states or conduction band
where Eg corresponds to the bandgap energy in either
energy (eV) or wavelength (nm).
1
the same time creation of a vacancy, h vb , in the valence band as illus-
trated in Figure 5.11.
The relative energy levels of the conduction band and valence band
states for some of the representative semiconductors listed in Tables 5.3
and 5.4 are shown in Figure 5.12. In the specific case of TiO , a semi-
2
conductor used in numerous commercial products, the conduction band
edge has a relative energy level at pH 0 of 0.4 V and the valence band
hole has an electrochemical potential of 2.8 V. Thus, in the case of TiO 2
the conduction band electron is mildly reducing although with suffi-
cient potential to reduce O to superoxide, O , while the valence band
2
2
hole at the surface of TiO is a powerful oxidizing agent.
2
For example, with use of the appropriate semiconductor catalysts, it
is possible to drive the photoelectrolysis of water into hydrogen and
oxygen as follows:
UV & visible light
2H 2 O h (18)
2H 2 1 O 2
The overall multi-electron redox potentials for the multi-electron reac-
tions at pH 7 suggest that H production with the input of UV-visible
2
light energy is feasible.
1
H O 4 H 1 OH 2 (19)
2
2
2H O 1 2 e 4 H 1 2 OH (20)
2
2
1
O 4 O 1 4H 1 4e (21)
2H 2 2
The redox potential for equation 21 at pH 7 is E H 1.23 V (NHE) with
the corresponding half-reactions of 0.41 V (Eq. 20) and 0.82 V (Eq. 21),