Page 179 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 179
164 Principles and Methods
– O 2 N NO 2
N + + N 4O • 4O 2
2
N N N N N N
N N N N N N N N H
H
OCH 3 CH O
3 OCH 3 CH O
3
NO 2 O N –
2 2CI 2HCI
NBT 4H + Formazan
Figure 5.8 NBT reduction by superoxide.
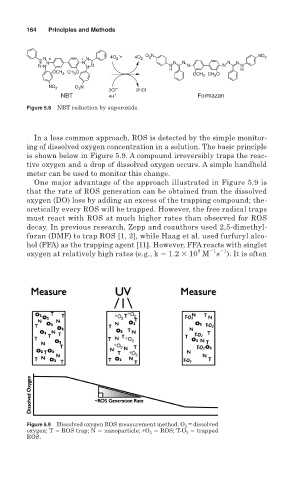
In a less common approach, ROS is detected by the simple monitor-
ing of dissolved oxygen concentration in a solution. The basic principle
is shown below in Figure 5.9. A compound irreversibly traps the reac-
tive oxygen and a drop of dissolved oxygen occurs. A simple handheld
meter can be used to monitor this change.
One major advantage of the approach illustrated in Figure 5.9 is
that the rate of ROS generation can be obtained from the dissolved
oxygen (DO) loss by adding an excess of the trapping compound; the-
oretically every ROS will be trapped. However, the free radical traps
must react with ROS at much higher rates than observed for ROS
decay. In previous research, Zepp and coauthors used 2,5-dimethyl-
furan (DMF) to trap ROS [1, 2], while Haag et al. used furfuryl alco-
hol (FFA) as the trapping agent [11]. However, FFA reacts with singlet
8 1 1
oxygen at relatively high rates (e.g., k 1.2
10 M s ). It is often
Figure 5.9 Dissolved oxygen ROS measurement method. O 2 = dissolved
oxygen; T ROS trap; N nanoparticle; ∗O 2 ROS; T-O 2 trapped
ROS.