Page 175 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 175
160 Principles and Methods
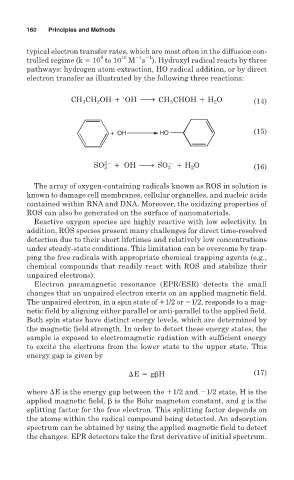
typical electron transfer rates, which are most often in the diffusion con-
1 1
10
9
trolled regime (k 10 to 10 M s ). Hydroxyl radical reacts by three
pathways: hydrogen atom extraction, HO radical addition, or by direct
electron transfer as illustrated by the following three reactions:
.
CHOH 1 H O
2
3
CH CH OH 1 OH h CH 3. 2 (14)
.
+ OH HO (15)
SO 22 . 2. (16)
3 1 OH h SO 3 1 H 2 O
The array of oxygen-containing radicals known as ROS in solution is
known to damage cell membranes, cellular organelles, and nucleic acids
contained within RNA and DNA. Moreover, the oxidizing properties of
ROS can also be generated on the surface of nanomaterials.
Reactive oxygen species are highly reactive with low selectivity. In
addition, ROS species present many challenges for direct time-resolved
detection due to their short lifetimes and relatively low concentrations
under steady-state conditions. This limitation can be overcome by trap-
ping the free radicals with appropriate chemical trapping agents (e.g.,
chemical compounds that readily react with ROS and stabilize their
unpaired electrons).
Electron paramagnetic resonance (EPR/ESR) detects the small
changes that an unpaired electron exerts on an applied magnetic field.
The unpaired electron, in a spin state of 1/2 or 1/2, responds to a mag-
netic field by aligning either parallel or anti-parallel to the applied field.
Both spin states have distinct energy levels, which are determined by
the magnetic field strength. In order to detect these energy states, the
sample is exposed to electromagnetic radiation with sufficient energy
to excite the electrons from the lower state to the upper state. This
energy gap is given by
E 5 g H (17)
where E is the energy gap between the 1/2 and 1/2 state, H is the
applied magnetic field, is the Böhr magneton constant, and g is the
splitting factor for the free electron. This splitting factor depends on
the atoms within the radical compound being detected. An adsorption
spectrum can be obtained by using the applied magnetic field to detect
the changes. EPR detectors take the first derivative of initial spectrum.