Page 172 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 172
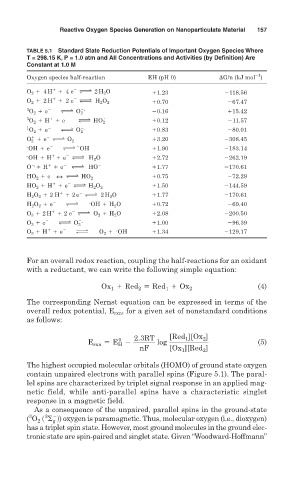
Reactive Oxygen Species Generation on Nanoparticulate Material 157
TABLE 5.1 Standard State Reduction Potentials of Important Oxygen Species Where
T = 298.15 K, P = 1.0 atm and All Concentrations and Activities (by Definition) Are
Constant at 1.0 M
1
Oxygen species half-reaction EH (pH 0) G/n (kJ mol )
2
1
O 2 1 4 H 1 4 e m 2 H 2 O 1.23 118.56
1 2
O 2 1 2 H 1 2 e m H 2 O 2 0.70 67.47
3 2 2.
O 2 1 e m O 2 0.16 15.42
3 1 2 . 0.12 11.57
O 2 1 H 1 e m HO 2
1 2 2.
O 2 1 e m O 2 0.83 80.01
1 2
O 2 1 e m O 2 3.20 308.45
. 2 2
OH 1 e m OH 1.90 183.14
. 1 2
OH 1 H 1 e m H 2 O 2.72 262.19
2. 1 2 2
O 1 H 1 e m HO 1.77 170.61
HO 2 1 e 2 4 m HO 2 2 0.75 72.29
1 2
HO 2 1 H 1 e m H 2 O 2 1.50 144.59
1
2
H 2 O 2 1 2 H 1 2 e m 2 H 2 O 1.77 170.61
.
2
H 2 O 2 1 e m OH 1 H 2 O 0.72 69.40
1
2
O 3 1 2 H 1 2 e m O 2 1 H 2 O 2.08 200.50
2 2.
O 3 1 e m O 3 1.00 96.39
#
2
1
O 3 1 H 1 e m O 2 1 OH 1.34 129.17
For an overall redox reaction, coupling the half-reactions for an oxidant
with a reductant, we can write the following simple equation:
(4)
Ox 1 1 Red 2 5 Red 1 1 Ox 2
The corresponding Nernst equation can be expressed in terms of the
overall redox potential, E rxn , for a given set of nonstandard conditions
as follows:
2.3RT [Red ][Ox 2 ]
1
0
E rxn 5 E H 2 log (5)
nF [Ox ][Red ]
2
1
The highest occupied molecular orbitals (HOMO) of ground state oxygen
contain unpaired electrons with parallel spins (Figure 5.1). The paral-
lel spins are characterized by triplet signal response in an applied mag-
netic field, while anti-parallel spins have a characteristic singlet
response in a magnetic field.
As a consequence of the unpaired, parallel spins in the ground-state
3 2
3
( O s g d ) oxygen is paramagnetic. Thus, molecular oxygen (i.e., dioxygen)
2
has a triplet spin state. However, most ground molecules in the ground elec-
tronic state are spin-paired and singlet state. Given “Woodward-Hoffmann”