Page 171 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 171
156 Principles and Methods
pathways will potentially produce reactive oxygen species in solution.
The net effect of both these processes is to convert light energy into oxi-
dizing chemical energy.
In engineered systems, oxidation serves many purposes, from elimi-
nation of potential hazards to improving aesthetic qualities. Chemical
oxidation may be used in a wide range of applications, such as the break-
down of organic compounds such as TCE and atrazine, the oxidation of
the reduced states of metals such as iron(II) and arsenic(III), or the
inactivation of pathogenic organisms in water treatment.
Oxidation is an electron transfer process in which electrons from a
reductant (i.e., an electron donor) are transferred to an oxidant and
electron acceptor. The thermodynamic constraints or boundary condi-
tions for electron transfer in aqueous solution are given by the electro-
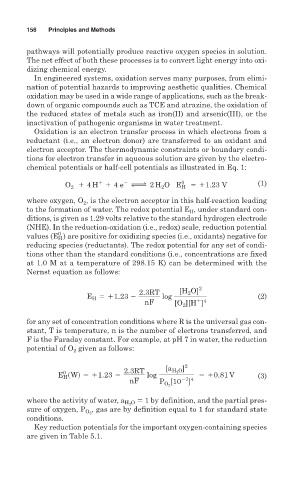
chemical potentials or half-cell potentials as illustrated in Eq. 1:
2
1
0
O 1 4 H 1 4 e m 2 H O E H 511.23 V (1)
2
2
where oxygen, O 2 , is the electron acceptor in this half-reaction leading
to the formation of water. The redox potential E H , under standard con-
ditions, is given as 1.29 volts relative to the standard hydrogen electrode
(NHE). In the reduction-oxidation (i.e., redox) scale, reduction potential
0
values (E H ) are positive for oxidizing species (i.e., oxidants) negative for
reducing species (reductants). The redox potential for any set of condi-
tions other than the standard conditions (i.e., concentrations are fixed
at 1.0 M at a temperature of 298.15 K) can be determined with the
Nernst equation as follows:
2.3RT [H O] 2
2
E H 511.23 2 log (2)
1 4
nF [O 2 ][H ]
for any set of concentration conditions where R is the universal gas con-
stant, T is temperature, n is the number of electrons transferred, and
F is the Faraday constant. For example, at pH 7 in water, the reduction
potential of O given as follows:
2
0
E H sWd 511.23 2 2.3RT log [a H 2 O ] 2 510.81 V (3)
27 4
nF P [10 ]
O 2
where the activity of water, a H 2 O 1 by definition, and the partial pres-
sure of oxygen, P , gas are by definition equal to 1 for standard state
O 2
conditions.
Key reduction potentials for the important oxygen-containing species
are given in Table 5.1.