Page 207 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 207
192 Principles and Methods
suspend fullerene in water. In each section, we will first discuss the
pathway specific properties of C 60 found in nonpolar solvents, following
with how encapsulation and functionalization of C 60 for aqueous sus-
pension affects that reaction group pathway.
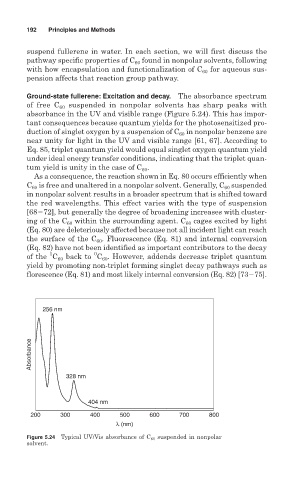
Ground-state fullerene: Excitation and decay. The absorbance spectrum
of free C 60 suspended in nonpolar solvents has sharp peaks with
absorbance in the UV and visible range (Figure 5.24). This has impor-
tant consequences because quantum yields for the photosensitized pro-
duction of singlet oxygen by a suspension of C 60 in nonpolar benzene are
near unity for light in the UV and visible range [61, 67]. According to
Eq. 85, triplet quantum yield would equal singlet oxygen quantum yield
under ideal energy transfer conditions, indicating that the triplet quan-
.
tum yield is unity in the case of C 60
As a consequence, the reaction shown in Eq. 80 occurs efficiently when
C is free and unaltered in a nonpolar solvent. Generally, C suspended
60
60
in nonpolar solvent results in a broader spectrum that is shifted toward
the red wavelengths. This effect varies with the type of suspension
[68 72], but generally the degree of broadening increases with cluster-
ing of the C 60 within the surrounding agent. C 60 cages excited by light
(Eq. 80) are deleteriously affected because not all incident light can reach
the surface of the C . Fluorescence (Eq. 81) and internal conversion
60
(Eq. 82) have not been identified as important contributors to the decay
1
0
of the C 60 back to C . However, addends decrease triplet quantum
60
yield by promoting non-triplet forming singlet decay pathways such as
florescence (Eq. 81) and most likely internal conversion (Eq. 82) [73 75].
256 nm
Absorbance
328 nm
404 nm
200 300 400 500 600 700 800
λ (nm)
Figure 5.24 Typical UV/Vis absorbance of C 60 suspended in nonpolar
solvent.