Page 211 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 211
196 Principles and Methods
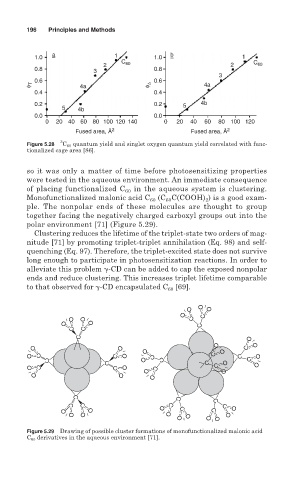
1.0 a 1 1.0 b 1
2 C 60 2 C 60
0.8 3 0.8
3
0.6 0.6
φ T 4a φ ∆ 4a
0.4 0.4
0.2 0.2 5 4b
5 4b
0.0 0.0
0 20 40 60 80 100 120 140 0 20 40 60 80 100 120
Fused area, Å 2 Fused area, Å 2
3
Figure 5.28 C 60 quantum yield and singlet oxygen quantum yield correlated with func-
tionalized cage area [86].
so it was only a matter of time before photosensitizing properties
were tested in the aqueous environment. An immediate consequence
of placing functionalized C 60 in the aqueous system is clustering.
Monofunctionalized malonic acid C 60 (C C(COOH) ) is a good exam-
2
60
ple. The nonpolar ends of these molecules are thought to group
together facing the negatively charged carboxyl groups out into the
polar environment [71] (Figure 5.29).
Clustering reduces the lifetime of the triplet-state two orders of mag-
nitude [71] by promoting triplet-triplet annihilation (Eq. 98) and self-
quenching (Eq. 97). Therefore, the triplet-excited state does not survive
long enough to participate in photosensitization reactions. In order to
alleviate this problem -CD can be added to cap the exposed nonpolar
ends and reduce clustering. This increases triplet lifetime comparable
to that observed for -CD encapsulated C 60 [69].
O
O
O
C
O
O C
O
O C
O
C
C
C O
O O O O C O
O
C O O C C C C C O
C O
C C O O
C
C O O C C O
C
O
O O
O
C
C C C C
C O
C
O C C
O
O
O
O O
O
O
O
O
O
Figure 5.29 Drawing of possible cluster formations of monofunctionalized malonic acid
C 60 derivatives in the aqueous environment [71].