Page 214 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 214
Reactive Oxygen Species Generation on Nanoparticulate Material 199
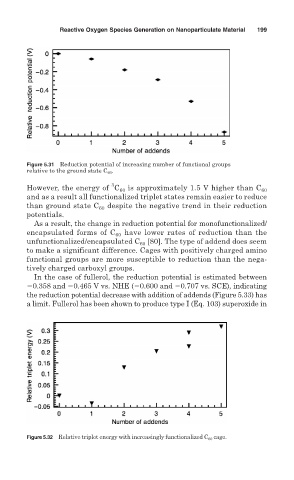
Figure 5.31 Reduction potential of increasing number of functional groups
relative to the ground state C 60 .
3
However, the energy of C 60 is approximately 1.5 V higher than C 60
and as a result all functionalized triplet states remain easier to reduce
than ground state C 60 despite the negative trend in their reduction
potentials.
As a result, the change in reduction potential for monofunctionalized/
encapsulated forms of C 60 have lower rates of reduction than the
unfunctionalized/encapsulated C 60 [80]. The type of addend does seem
to make a significant difference. Cages with positively charged amino
functional groups are more susceptible to reduction than the nega-
tively charged carboxyl groups.
In the case of fullerol, the reduction potential is estimated between
0.358 and 0.465 V vs. NHE ( 0.600 and 0.707 vs. SCE), indicating
the reduction potential decrease with addition of addends (Figure 5.33) has
a limit. Fullerol has been shown to produce type I (Eq. 103) superoxide in
Figure 5.32 Relative triplet energy with increasingly functionalized C 60 cage.