Page 215 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 215
200 Principles and Methods
3
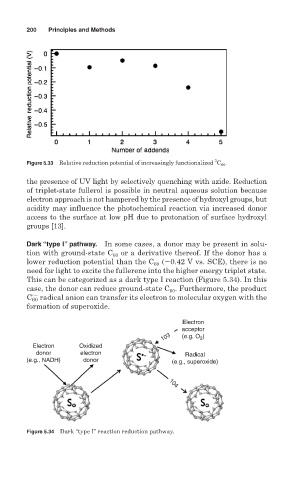
Figure 5.33 Relative reduction potential of increasingly functionalized C 60 .
the presence of UV light by selectively quenching with azide. Reduction
of triplet-state fullerol is possible in neutral aqueous solution because
electron approach is not hampered by the presence of hydroxyl groups, but
acidity may influence the photochemical reaction via increased donor
access to the surface at low pH due to protonation of surface hydroxyl
groups [13].
Dark “type I” pathway. In some cases, a donor may be present in solu-
tion with ground-state C 60 or a derivative thereof. If the donor has a
lower reduction potential than the C 60 ( 0.42 V vs. SCE), there is no
need for light to excite the fullerene into the higher energy triplet state.
This can be categorized as a dark type I reaction (Figure 5.34). In this
case, the donor can reduce ground-state C 60 . Furthermore, the product
?2
C 60 radical anion can transfer its electron to molecular oxygen with the
formation of superoxide.
Electron
acceptor
103 (e.g. O )
2
Electron Oxidized
donor electron Radical
(e.g., NADH) donor 104
(e.g., superoxide)
Figure 5.34 Dark “type I” reaction reduction pathway.