Page 212 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 212
Reactive Oxygen Species Generation on Nanoparticulate Material 197
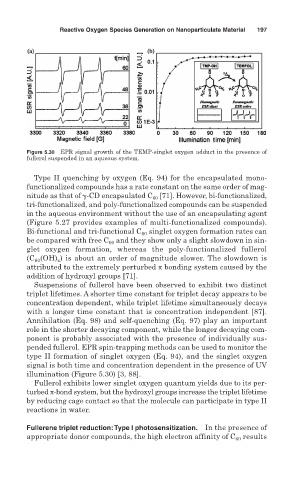
Figure 5.30 EPR signal growth of the TEMP-singlet oxygen adduct in the presence of
fullerol suspended in an aqueous system.
Type II quenching by oxygen (Eq. 94) for the encapsulated mono-
functionalized compounds has a rate constant on the same order of mag-
nitude as that of -CD encapsulated C [71]. However, bi-functionalized,
60
tri-functionalized, and poly-functionalized compounds can be suspended
in the aqueous environment without the use of an encapsulating agent
(Figure 5.27 provides examples of multi-functionalized compounds).
Bi-functional and tri-functional C singlet oxygen formation rates can
60
be compared with free C and they show only a slight slowdown in sin-
60
glet oxygen formation, whereas the poly-functionalized fullerol
(C (OH) ) is about an order of magnitude slower. The slowdown is
x
60
attributed to the extremely perturbed bonding system caused by the
addition of hydroxyl groups [71].
Suspensions of fullerol have been observed to exhibit two distinct
triplet lifetimes. A shorter time constant for triplet decay appears to be
concentration dependent, while triplet lifetime simultaneously decays
with a longer time constant that is concentration independent [87].
Annihilation (Eq. 98) and self-quenching (Eq. 97) play an important
role in the shorter decaying component, while the longer decaying com-
ponent is probably associated with the presence of individually sus-
pended fullerol. EPR spin-trapping methods can be used to monitor the
type II formation of singlet oxygen (Eq. 94), and the singlet oxygen
signal is both time and concentration dependent in the presence of UV
illumination (Figure 5.30) [3, 88].
Fullerol exhibits lower singlet oxygen quantum yields due to its per-
turbed -bond system, but the hydroxyl groups increase the triplet lifetime
by reducing cage contact so that the molecule can participate in type II
reactions in water.
Fullerene triplet reduction:Type I photosensitization. In the presence of
results
appropriate donor compounds, the high electron affinity of C 60