Page 256 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 256
Nanoparticle Transport, Aggregation, and Deposition 241
should favor extension of ordered water further into the bulk. Although
there is some controversy regarding the extent to which water may
extend into the bulk, it appears that at least two to three layers of
ordered water are likely present at most hydrated surfaces. As two
hydrated surfaces approach one another, dehydration must occur before
the underlying surfaces are in direct contact. The additional free energy
required for dehydration represents a repulsive barrier between the
two approaching surfaces. Hydration forces act over a shorter range
[decay length ( ) 0.2–1.1 nm] than attractive hydrophobic interactions
and decay exponentially with separation distance.
AB
The acid-base interaction energy (U ) between a sphere and a flat
surface is predicted to decay exponentially with separation distance
according to the following expression:
y 2 h
AB AB 0
l
U mlc shd 5 2pa p l G y 0 exp c d (6)
AB
where G y 0 is the acid-base free energy of interaction at contact; is
the characteristic decay length of AB interactions in water, whose value
is between 0.2 and 1.0 nm, a value of 0.6 nm is commonly used [3, 4].
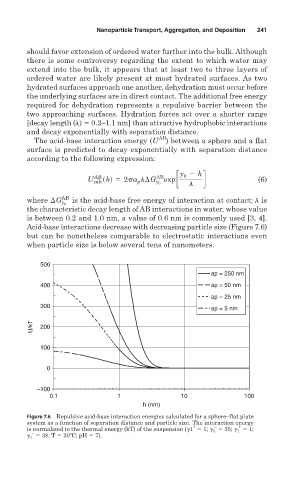
Acid-base interactions decrease with decreasing particle size (Figure 7.6)
but can be nonetheless comparable to electrostatic interactions even
when particle size is below several tens of nanometers.
500
ap = 250 nm
400 ap = 50 nm
ap = 25 nm
300 ap = 5 nm
U/kT 200
100
0
–100
0.1 1 10 100
h (nm)
Figure 7.6 Repulsive acid-base interaction energies calculated for a sphere–flat plate
system as a function of separation distance and particle size. The interaction energy
+ – +
is normalized to the thermal energy (kT) of the suspension ( 1 1; 1 35; 1 1;
–
1 38; T 20ºC; pH 7).