Page 259 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 259
244 Principles and Methods
Suspensions of nanoparticles may be stabilized through selection of
solvent polarity [28–32] and solution ionic strength [33, 34]. For
processes that require working in aqueous or water-based systems (that
may also be more representative of natural systems), manipulating sol-
vent polarity may be problematic and eventually impractical. A variety
of techniques have been developed for dispersing particles into suspen-
sion, including: solvent exchange for buckminsterfullerene [15, 33, 35],
extended agitation (and presumably slow reaction) [18, 36], wrapping
or grafting molecules, such as surfactants, that impart hydrophilicity
to the nanoparticles [37], or direct functionalization of the nanoparticle
to produce hydrophilic groups.
Aggregation kinetics and particle stability
The stability of particle dispersions may be evaluated by comparing the
aggregation rates of “sticky” and less sticky particles. Here, the sticki-
ness is accounted for in terms of a collision efficiency [38]. In the case
of perfectly sticky particles ( 1), aggregation is assumed to be lim-
ited only by the transport of particles up to one another. The ratio of the
aggregation rates between these two cases is characterized in terms of
a stability ratio, W, which may be written as:
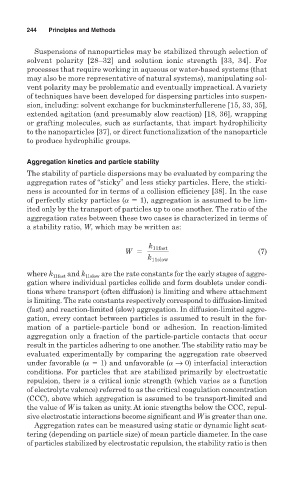
k 11fast
W 5 (7)
k 11slow
where k 11fast and k 11slow are the rate constants for the early stages of aggre-
gation where individual particles collide and form doublets under condi-
tions where transport (often diffusion) is limiting and where attachment
is limiting. The rate constants respectively correspond to diffusion-limited
(fast) and reaction-limited (slow) aggregation. In diffusion-limited aggre-
gation, every contact between particles is assumed to result in the for-
mation of a particle-particle bond or adhesion. In reaction-limited
aggregation only a fraction of the particle-particle contacts that occur
result in the particles adhering to one another. The stability ratio may be
evaluated experimentally by comparing the aggregation rate observed
under favorable ( 1) and unfavorable ( → 0) interfacial interaction
conditions. For particles that are stabilized primarily by electrostatic
repulsion, there is a critical ionic strength (which varies as a function
of electrolyte valence) referred to as the critical coagulation concentration
(CCC), above which aggregation is assumed to be transport-limited and
the value of W is taken as unity. At ionic strengths below the CCC, repul-
sive electrostatic interactions become significant and W is greater than one.
Aggregation rates can be measured using static or dynamic light scat-
tering (depending on particle size) of mean particle diameter. In the case
of particles stabilized by electrostatic repulsion, the stability ratio is then