Page 263 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 263
248 Principles and Methods
volume concentration, the number concentration of particles, n decreases
j
–1
3
3
inversely proportional to r . The (nm s ) coefficient is only depend-
j
jj
ent on the particle volume or size when other environmental parameters
are kept constant. When r is sufficiently high so that the Brownian con-
j
tribution ( jjBr 8kT ) is negligible with regard to the mechanical shear-
3m
32 3
ing contribution ( jj sh r G m ) (eq. 14), then varies as r . Conversely,
3
3 j
jj
j
as particle size decreases, the contribution from Brownian motion
becomes significant and induces a horizontal asymptote at jj (rj → 0) ≈ 8kT .
3m
–1
In Figure 7.7, the magnitude of the mean shear rate (G 100 s ) is rep-
m
resentative of conditions in natural systems. Under these conditions
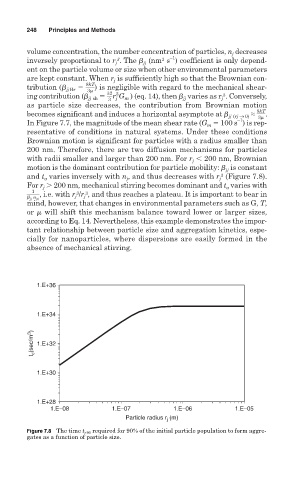
Brownian motion is significant for particles with a radius smaller than
200 nm. Therefore, there are two diffusion mechanisms for particles
with radii smaller and larger than 200 nm. For r 200 nm, Brownian
j
motion is the dominant contribution for particle mobility: is constant
jj
3
and t varies inversely with n , and thus decreases with r (Figure 7.8).
j
j
a
For r 200 nm, mechanical stirring becomes dominant and t varies with
j
a
1
, i.e. with r /r , and thus reaches a plateau. It is important to bear in
3
3
j
j
b jj n j0
mind, however, that changes in environmental parameters such as G, T,
or will shift this mechanism balance toward lower or larger sizes,
according to Eq. 14. Nevertheless, this example demonstrates the impor-
tant relationship between particle size and aggregation kinetics, espe-
cially for nanoparticles, where dispersions are easily formed in the
absence of mechanical stirring.
1.E+36
1.E+34
t c (sec/m 3 ) 1.E+32
1.E+30
1.E+28
1.E–08 1.E–07 1.E–06 1.E–05
(m)
Particle radius r j
Figure 7.8 The time t c90 required for 90% of the initial particle population to form aggre-
gates as a function of particle size.