Page 265 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 265
250 Principles and Methods
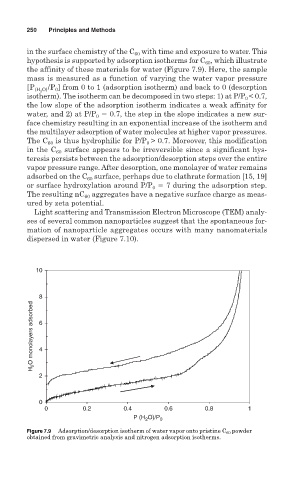
in the surface chemistry of the C with time and exposure to water. This
60
hypothesis is supported by adsorption isotherms for C , which illustrate
60
the affinity of these materials for water (Figure 7.9). Here, the sample
mass is measured as a function of varying the water vapor pressure
[P (H 2 O) /P ] from 0 to 1 (adsorption isotherm) and back to 0 (desorption
0
isotherm). The isotherm can be decomposed in two steps: 1) at P/P < 0.7,
0
the low slope of the adsorption isotherm indicates a weak affinity for
water, and 2) at P/P 0.7, the step in the slope indicates a new sur-
0
face chemistry resulting in an exponential increase of the isotherm and
the multilayer adsorption of water molecules at higher vapor pressures.
The C 60 is thus hydrophilic for P/P > 0.7. Moreover, this modification
0
in the C 60 surface appears to be irreversible since a significant hys-
teresis persists between the adsorption/desorption steps over the entire
vapor pressure range. After desorption, one monolayer of water remains
adsorbed on the C surface, perhaps due to clathrate formation [15, 19]
60
or surface hydroxylation around P/P 7 during the adsorption step.
0
The resulting nC aggregates have a negative surface charge as meas-
60
ured by zeta potential.
Light scattering and Transmission Electron Microscope (TEM) analy-
ses of several common nanoparticles suggest that the spontaneous for-
mation of nanoparticle aggregates occurs with many nanomaterials
dispersed in water (Figure 7.10).
10
8
H 2 O monolayers adsorbed 6 4
2
0
0 0.2 0.4 0.6 0.8 1
P (H O)/P 0
2
Figure 7.9 Adsorption/desorption isotherm of water vapor onto pristine C 60 powder
obtained from gravimetric analysis and nitrogen adsorption isotherms.