Page 266 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 266
Nanoparticle Transport, Aggregation, and Deposition 251
This may be attributed in part to a decreasing electrostatic repulsion
between charged particles with decreasing size for those particles that
develop a charge in water. This topic was covered earlier in this chapter
showing the influence of particle size on the interfacial energy for par-
ticles in water (Figure 7.6). The size, structure, and chemical properties
of these clusters are dependent on the characteristics of the constituent
nanoparticles [47] and the process by which the particles are put into sus-
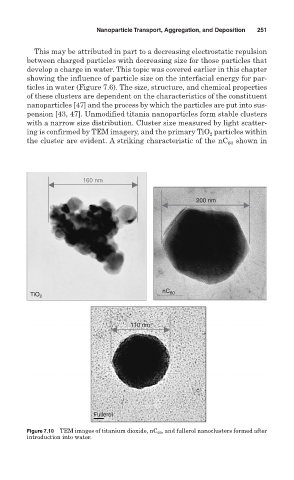
pension [43, 47]. Unmodified titania nanoparticles form stable clusters
with a narrow size distribution. Cluster size measured by light scatter-
ing is confirmed by TEM imagery, and the primary TiO particles within
2
the cluster are evident. A striking characteristic of the nC 60 shown in
160 nm
200 nm
nC
TiO 2 60
110 nm
Fullerol
Figure 7.10 TEM images of titanium dioxide, nC 60 , and fullerol nanoclusters formed after
introduction into water.