Page 264 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 264
Nanoparticle Transport, Aggregation, and Deposition 249
We can continue our theoretical consideration of aggregation to
include calculations of the stickiness coefficient, or its reciprocal, W.
The stability ratio can be calculated from theory as an integral meas-
ure of the interaction potential between two particles:
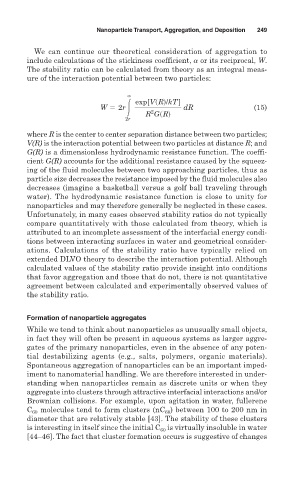
`
exp[VsRd/kT ]
W 5 2r dR (15)
R GsRd
3 2
2r
where R is the center to center separation distance between two particles;
V(R) is the interaction potential between two particles at distance R; and
G(R) is a dimensionless hydrodynamic resistance function. The coeffi-
cient G(R) accounts for the additional resistance caused by the squeez-
ing of the fluid molecules between two approaching particles, thus as
particle size decreases the resistance imposed by the fluid molecules also
decreases (imagine a basketball versus a golf ball traveling through
water). The hydrodynamic resistance function is close to unity for
nanoparticles and may therefore generally be neglected in these cases.
Unfortunately, in many cases observed stability ratios do not typically
compare quantitatively with those calculated from theory, which is
attributed to an incomplete assessment of the interfacial energy condi-
tions between interacting surfaces in water and geometrical consider-
ations. Calculations of the stability ratio have typically relied on
extended DLVO theory to describe the interaction potential. Although
calculated values of the stability ratio provide insight into conditions
that favor aggregation and those that do not, there is not quantitative
agreement between calculated and experimentally observed values of
the stability ratio.
Formation of nanoparticle aggregates
While we tend to think about nanoparticles as unusually small objects,
in fact they will often be present in aqueous systems as larger aggre-
gates of the primary nanoparticles, even in the absence of any poten-
tial destabilizing agents (e.g., salts, polymers, organic materials).
Spontaneous aggregation of nanoparticles can be an important imped-
iment to nanomaterial handling. We are therefore interested in under-
standing when nanoparticles remain as discrete units or when they
aggregate into clusters through attractive interfacial interactions and/or
Brownian collisions. For example, upon agitation in water, fullerene
C 60 molecules tend to form clusters (nC ) between 100 to 200 nm in
60
diameter that are relatively stable [43]. The stability of these clusters
is interesting in itself since the initial C is virtually insoluble in water
60
[44–46]. The fact that cluster formation occurs is suggestive of changes