Page 254 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 254
Nanoparticle Transport, Aggregation, and Deposition 239
500
ap = 250 nm
ap = 50 nm
400
ap = 25 nm
ap = 5 nm
300
U/kT
200
100
0
0 1 10
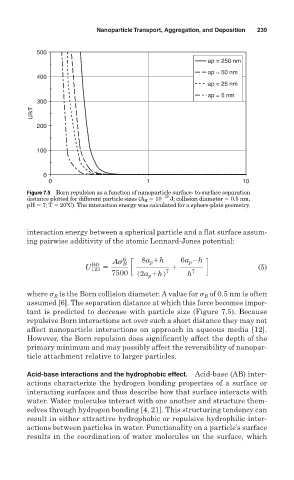
Figure 7.5 Born repulsion as a function of nanoparticle surface- to-surface separation
distance plotted for different particle sizes (A H 10 20 J; collision diameter 0.5 nm;
pH 7; T 20ºC). The interaction energy was calculated for a sphere-plate geometry.
interaction energy between a spherical particle and a flat surface assum-
ing pairwise additivity of the atomic Lennard-Jones potential:
As 6 B 8a 1h 6a 2h
p
p
U BO 5 c 1 d (5)
123
7500 s2a 1hd 7 h 7
p
where is the Born collision diameter. A value for of 0.5 nm is often
B
B
assumed [6]. The separation distance at which this force becomes impor-
tant is predicted to decrease with particle size (Figure 7.5). Because
repulsive Born interactions act over such a short distance they may not
affect nanoparticle interactions on approach in aqueous media [12].
However, the Born repulsion does significantly affect the depth of the
primary minimum and may possibly affect the reversibility of nanopar-
ticle attachment relative to larger particles.
Acid-base interactions and the hydrophobic effect. Acid-base (AB) inter-
actions characterize the hydrogen bonding properties of a surface or
interacting surfaces and thus describe how that surface interacts with
water. Water molecules interact with one another and structure them-
selves through hydrogen bonding [4, 21]. This structuring tendency can
result in either attractive hydrophobic or repulsive hydrophilic inter-
actions between particles in water. Functionality on a particle’s surface
results in the coordination of water molecules on the surface, which