Page 253 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 253
238 Principles and Methods
30
20 CaCl 2
10 NaCl
0
ζ(mV) –10
–20
–30
–40
–50
–60
0.00001 0.0001 0.001 0.01 0.1
Salt concentration (M)
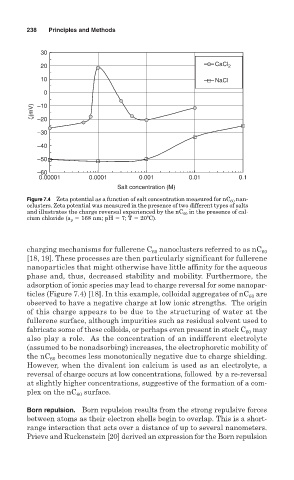
Figure 7.4 Zeta potential as a function of salt concentration measured for nC 60 nan-
oclusters. Zeta potential was measured in the presence of two different types of salts
and illustrates the charge reversal experienced by the nC 60 in the presence of cal-
cium chloride (a p 168 nm; pH 7; T 20ºC).
charging mechanisms for fullerene C 60 nanoclusters referred to as nC 60
[18, 19]. These processes are then particularly significant for fullerene
nanoparticles that might otherwise have little affinity for the aqueous
phase and, thus, decreased stability and mobility. Furthermore, the
adsorption of ionic species may lead to charge reversal for some nanopar-
ticles (Figure 7.4) [18]. In this example, colloidal aggregates of nC 60 are
observed to have a negative charge at low ionic strengths. The origin
of this charge appears to be due to the structuring of water at the
fullerene surface, although impurities such as residual solvent used to
fabricate some of these colloids, or perhaps even present in stock C 60 may
also play a role. As the concentration of an indifferent electrolyte
(assumed to be nonadsorbing) increases, the electrophoretic mobility of
the nC 60 becomes less monotonically negative due to charge shielding.
However, when the divalent ion calcium is used as an electrolyte, a
reversal of charge occurs at low concentrations, followed by a re-reversal
at slightly higher concentrations, suggestive of the formation of a com-
plex on the nC 60 surface.
Born repulsion. Born repulsion results from the strong repulsive forces
between atoms as their electron shells begin to overlap. This is a short-
range interaction that acts over a distance of up to several nanometers.
Prieve and Ruckenstein [20] derived an expression for the Born repulsion