Page 252 - Environmental Nanotechnology Applications and Impacts of Nanomaterials
P. 252
Nanoparticle Transport, Aggregation, and Deposition 237
200
ap = 250 nm
180
ap = 50 nm
160
ap = 25 nm
140
ap = 5 nm
120
U/kT 100
80
60
40
20
0
0 1 10 100
h (nm)
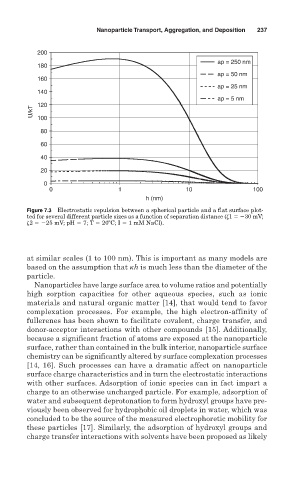
Figure 7.3 Electrostatic repulsion between a spherical particle and a flat surface plot-
ted for several different particle sizes as a function of separation distance ( 1 30 mV;
2 25 mV; pH 7; T 20ºC; I 1 mM NaCl).
at similar scales (1 to 100 nm). This is important as many models are
based on the assumption that h is much less than the diameter of the
particle.
Nanoparticles have large surface area to volume ratios and potentially
high sorption capacities for other aqueous species, such as ionic
materials and natural organic matter [14], that would tend to favor
complexation processes. For example, the high electron-affinity of
fullerenes has been shown to facilitate covalent, charge transfer, and
donor-acceptor interactions with other compounds [15]. Additionally,
because a significant fraction of atoms are exposed at the nanoparticle
surface, rather than contained in the bulk interior, nanoparticle surface
chemistry can be significantly altered by surface complexation processes
[14, 16]. Such processes can have a dramatic affect on nanoparticle
surface charge characteristics and in turn the electrostatic interactions
with other surfaces. Adsorption of ionic species can in fact impart a
charge to an otherwise uncharged particle. For example, adsorption of
water and subsequent deprotonation to form hydroxyl groups have pre-
viously been observed for hydrophobic oil droplets in water, which was
concluded to be the source of the measured electrophoretic mobility for
these particles [17]. Similarly, the adsorption of hydroxyl groups and
charge transfer interactions with solvents have been proposed as likely